MULTISCREEN™ Beta Arrestin Sensor Technology
High Throughput Detection of Biased Cell Signaling of Unmodified GPCRs
What is Beta Arrestin?
Beta-arrestin is a family of proteins involved the regulation of signaling and trafficking for GPCRs through desensitization. They can be activated by a wide range of ligands, from light-sensitive compounds to hormones and neurotransmitters. Following GPCR activation by external signals, protein activation and initiation of a signaling cascade results from its interaction with a heterotrimeric G protein. The receptor then undergoes phosphorylation by G protein-coupled receptor kinases (GRKs) to prevent prolonged or inappropriate signaling, while also marking the receptor for binding by proteins like beta-arrestin.
Beta-arrestin binds to the phosphorylated receptor, further inhibiting GPCR activity by preventing interactions with G proteins, while also tagging the receptor for internalization into the cell. This interaction removes the activated receptor from the cell surface, dampening the cellular response to the external signal.
Beta-arrestins are also involved in signaling pathways themselves, acting as scaffolds by organizing other signaling proteins and initiating distinct pathways independent of G proteins. There are also other forms of arrestins, including visual arrestins and cone arrestins, which are specifically involved in the rapid and highly regulated response of photoreceptor cells to light.
The study of beta-arrestins in live cells is essential in understanding their interactions with GPCRs, providing deeper insights into cellular communication and protein networks, and underscoring their potential as targets for therapeutic intervention in a variety of diseases where GPCR activity is dysregulated.
The bifurcation of GPCR signal transduction between G proteins and arrestins has attracted major interest in academic and pharmaceutical research on target-related pathogenesis in recent years. More importantly, possibilities of designing compounds that preferentially activate or inhibit specific GPCR signal transduction pathways have brought new hope for finding efficacious therapies without harmful side effects to the drug discovery community.
Why MULTISCREEN™ Beta Arrestin Sensor Technology?
There have been a number of established technologies used to measure GPCR β-arrestin signaling. These older screening assays that can be used in high throughput screening all require tagging GPCRs intracellularly at the C-terminus with an enzyme, enzyme fragment, or fluorescence protein. As C-terminus of GPCRs are the functional domains used for arrestin binding and G protein binding, these sizable protein tags can themselves cause biases in receptor signaling by altering protein-protein interactions, confounding the analysis of experimental results. Furthermore, some studies resort to using one cell line with unmodified GPCR for G protein pathway analysis and tagged receptor in another cell line for arrestin analysis. This approach further introduces unwanted biases generated from different cell lines, which are irrelevant to the target GPCR-triggered cell signaling. Finally, the requirement of GPCR tagging from the older screening assays makes studies of endogenous GPCRs completely unattainable. The need to overcome these limitations has led to the genesis of Multispan’s MULTISCREEN™ Technology, specifically designed to measure β-Arrestin recruitment by unmodified GPCRs.
How does it work?
MULTISCREEN™ Technology employs a bicistronic mammalian expression vector that carries 2 fusion proteins:
- β-Arrestin fused to the N-terminal luciferase fragment
- A membrane anchor fused to the C-terminal luciferase fragment
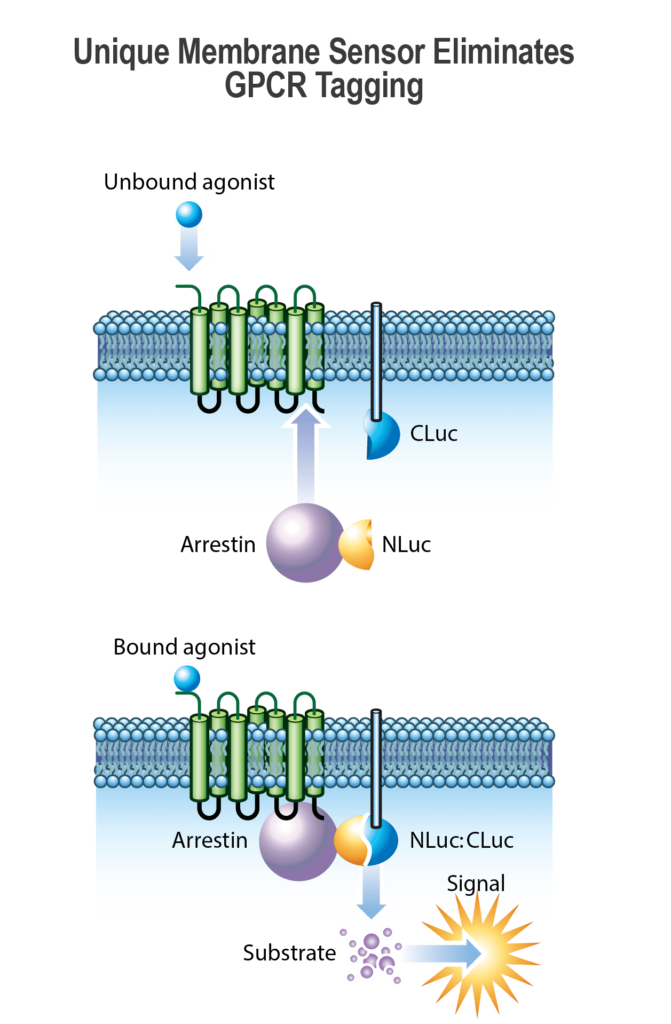
The luciferase enzyme fragments are proprietary whereas the membrane anchor protein is ubiquitously expressed on the cellular plasma membrane. This bicistronic insert has also been cloned into a MacMam viral expression vector for robust transient expression of the β-Arrestin sensor in a variety of cell lines.
In cells co-expressing the MULTISCREEN™ Sensor and an endogenous or exogenous unmodified GPCR target, the activation of GPCR by its agonists leads to the recruitment of arrestins to the GPCR on the cell membrane. The proximity of the two fusion proteins lead to the reconstitution of the whole luciferase enzyme activity through complementation, which can be detected by a luminescence substrate. The signal can be read conveniently by standard microplate readers.
MULTISCREEN™ Stable Cell Line
Using this technology, we were able to generate HTS assay-ready stable cell lines for a variety of GPCRs. For example, we developed stable cell lines for all 4 opioid receptors. Specifically, the MULTISCREEN™ Sensor expressed in MOR stable cell line clearly differentiated efficacies of super agonist DAMGO, full agonist Morphine and partial agonist Buprenorphine. In addition, the MOR β-arrestin recruitment assay gave >0.7 Z’ for high quality high throughput screening.
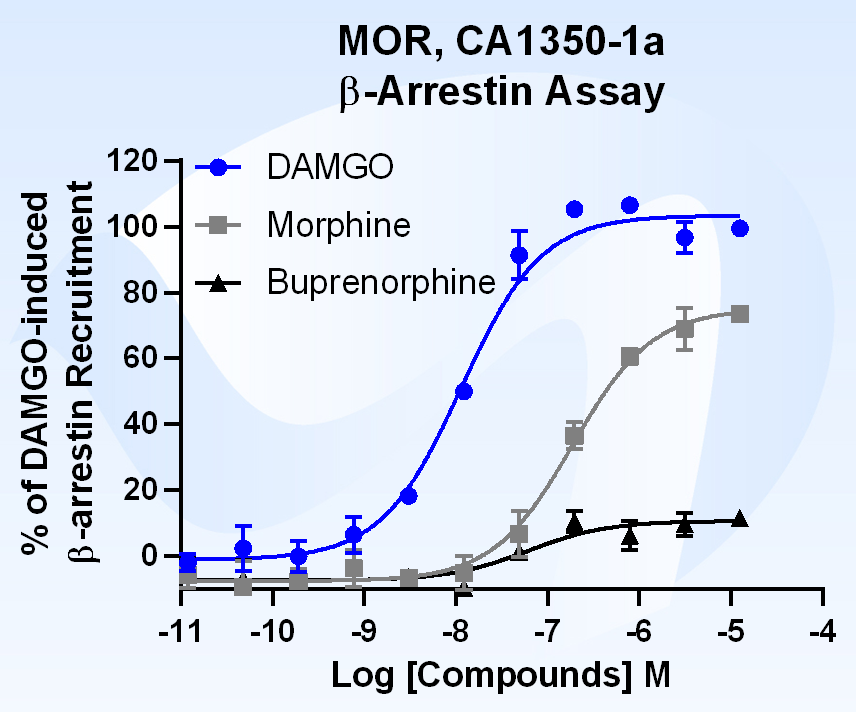
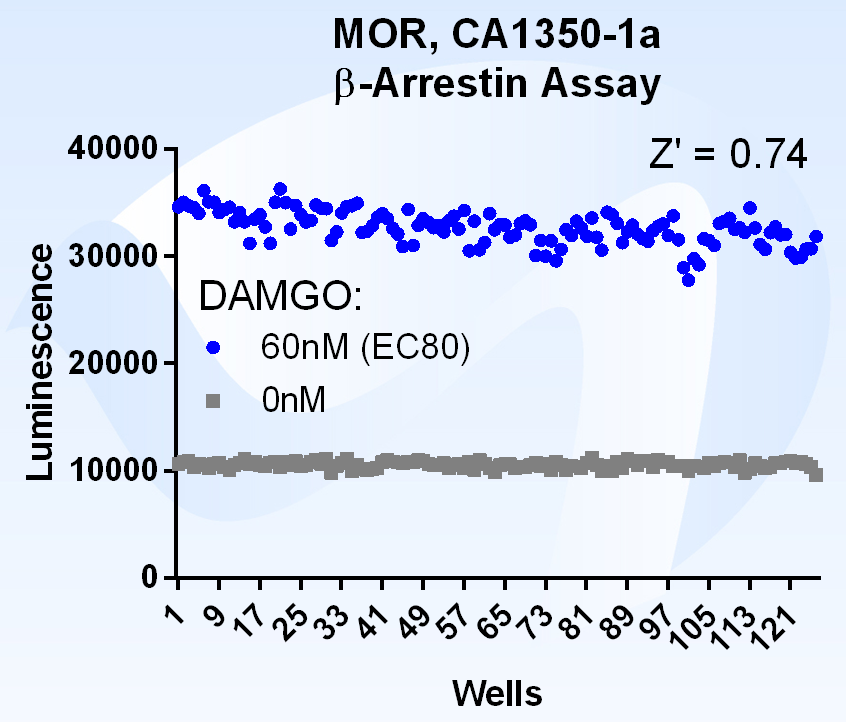
Two Assays in One Well
Leveraging the differences between β-arrestin and cAMP signaling kinetics and assay readouts, we developed high throughput and multiplexed screening methods for both assays in the same cell line and the same well. The dose-response curves from MULTISCREEN™ MOR stable cell line below were generated from such multiplexed assay format: β-arrestin luminescence assay was read first followed by TR-FRET cAMP assay as the 2nd readout.
“Two Assays in One Well” provides added advantage to MULTISCREEN™ Technology in signaling bias compound profiling by eliminating the one more well-to-well variable. In addition to high quality data, while “Two Assays in One Well” also provides 50% reduction of time and cost of materials related to cells, media, plates and supplies.
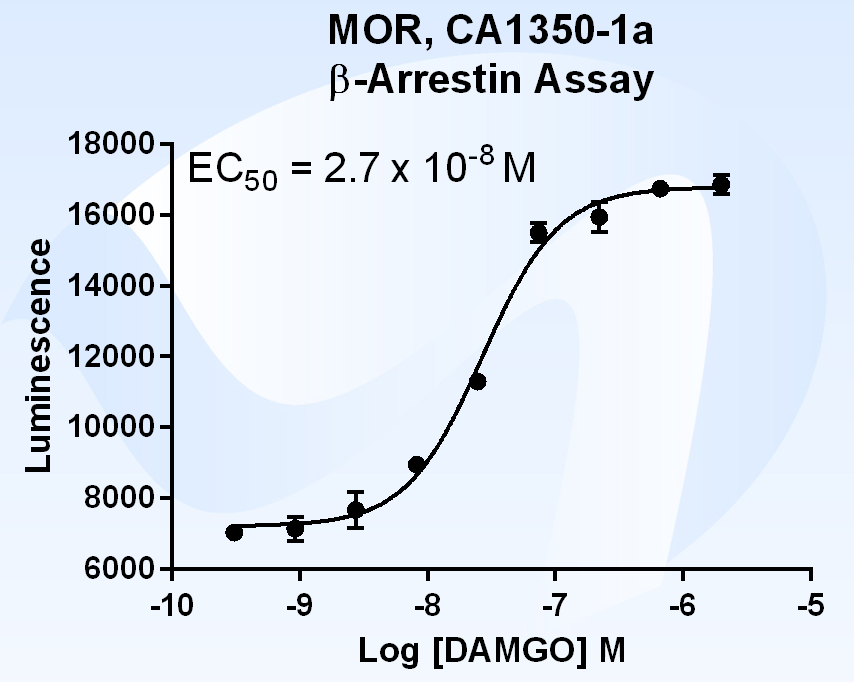
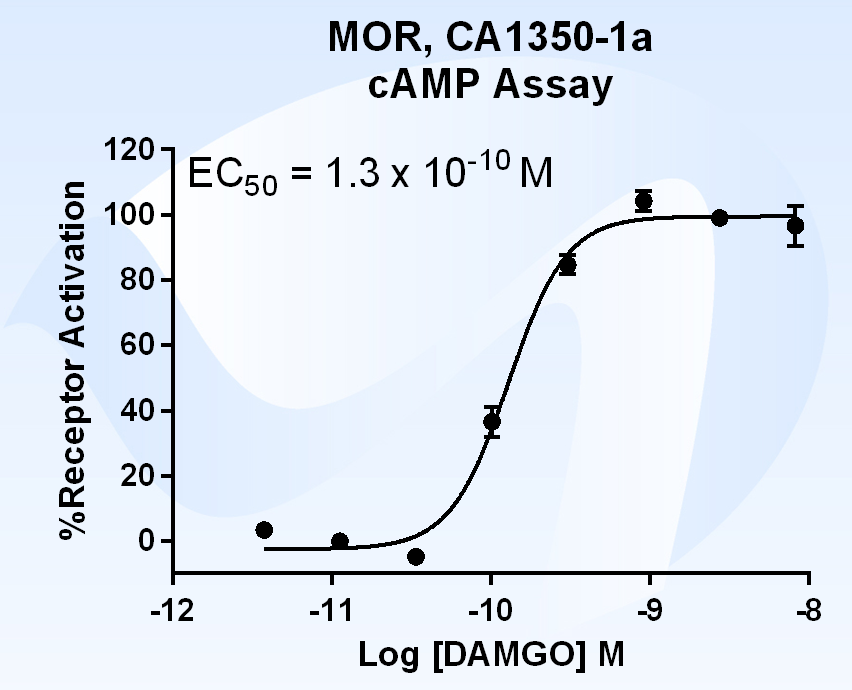
BacMam
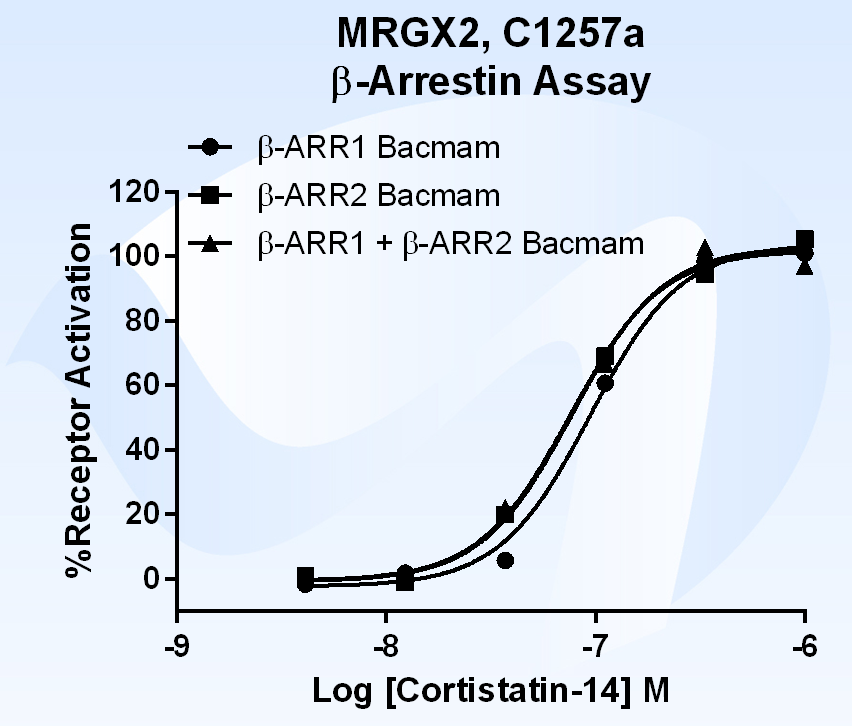
Besides using MULTISCREEN™ stable cell lines co-expressing GPCR and β-arrestin, we developed proprietary BacMam viral expression vector that enables robust transient expression of the sensor in recombinant or native cells. It proves to be simpler, less expensive, faster, and gentler than the various traditional transient transfection methods. By way of example, MULTISCREEN™ MRGX2 Stable Cell Line was transduced with β-Arrestin1 and β-Arrestin2 BacMam viral particles and assayed robustly in the MULTISCREEN™ recruitment assay 48 hours later.
The First HTS Beta-Arrestin Assay for Native GPCRs in Recombinant or Primary Cells
- No GPCR tagging required – assess true receptor pharmacology
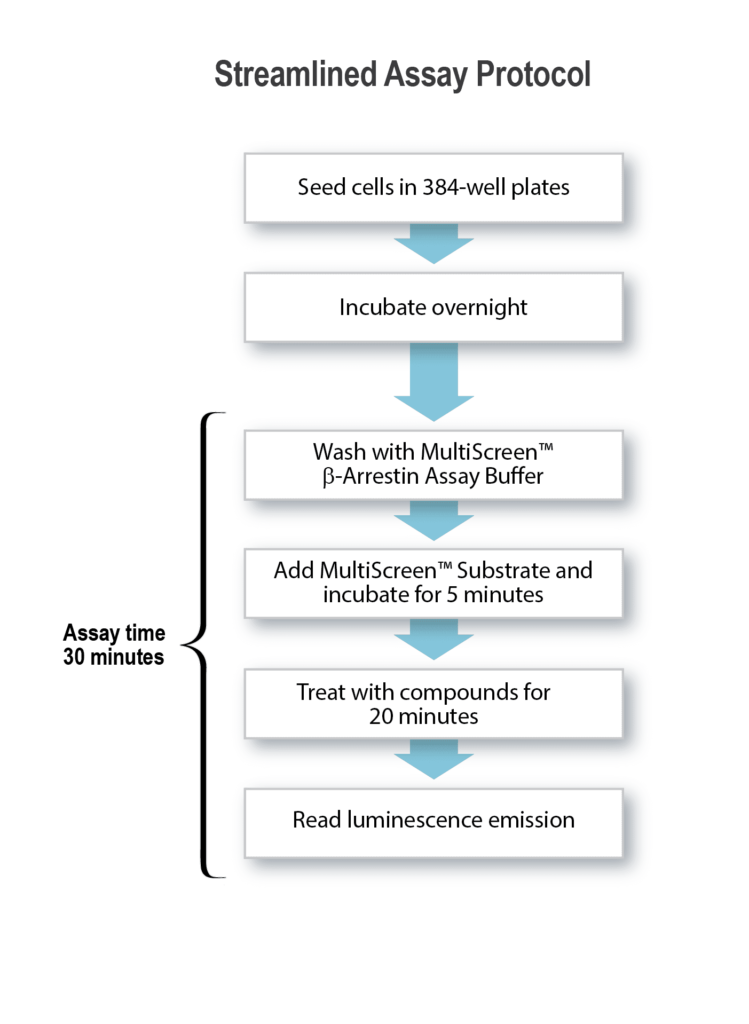
- No wash, homogeneous assay in 96- or 384-well microplate formats
- Fully validated for transiently or stably transfected cells
- No GRK2 co-transfection required
- Available as assay kits, reagents, cell lines, and services
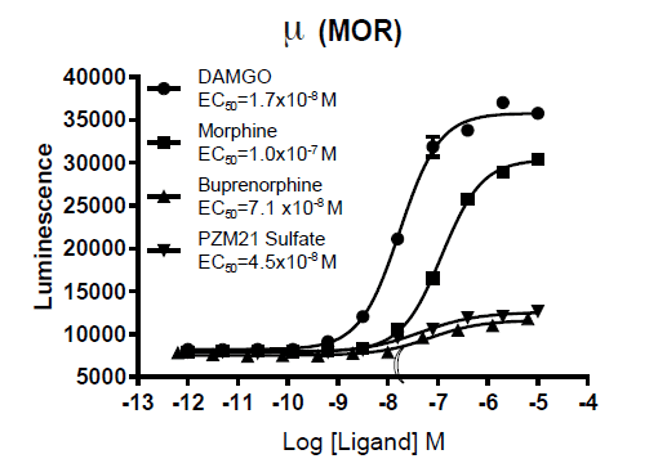
CHO-K1 cells stably expressing the µ opioid receptor were stimulated with known agonists and the MultiScreen β-arrestin assay run in a 384-well format.
Developing Candidates with Enhanced Efficacy & Safety Profiles
With the expanding importance of Arrestin-mediated signaling and the role of biased signaling in GPCR physiology, robust β-Arrestin assays have become a critical piece of GPCR-targeted drug development.
First generation assays rely on tagging both the GPCR-of-interest and β-Arrestin with fusion proteins that when brought together mediate production of light. Multispan’s proprietary MULTISCREENTM sensor technology relies on unmodified GPCRS and thus overcomes receptor-tagging drawback of first-generation technologies, enabling high-throughput detection of β-arrestin translocation induced by native GPCRs in vitro and in vivo.
Don’t let GPCR tagging bias your results. Find out how to unbias your GPCR signaling research.
Pioneering Next-Gen GPCR Assays
Our assays utilize the translocation of β-Arrestin for the assay readout, a process distinct due to the tagging of β-Arrestin and a membrane sensor instead of the GPCR itself. This approach facilitates closer proximity between the tagged arrestin and the membrane sensor, initiating a functional luciferase enzyme reaction.
By avoiding GPCR tagging, it sidesteps the associated drawbacks, offering a viable method to study β-Arrestin activation in endogenous or orphan GPCRs. This technique is designed for high throughput, cell-based screening, promising efficiency and accuracy in GPCR research.
For detailed insights, consult with our technical team or request a quote.
Why are Unmodified GPCRs so Important?
In the highly specialized domain of GPCR-targeted drug development, the importance of working with unmodified GPCRs cannot be overstated. Traditional β-Arrestin assays necessitate GPCR tagging, a process that has been shown to potentially induce deleterious receptor conformational changes, promote steric hindrance, or even alter receptor pharmacology. Such modifications not only compromise the physiological relevance of the data generated but can significantly impact the identification and optimization of highly qualified drug candidates.
Recognizing these challenges, the advanced MULTISCREENTM Technology stands out as a reliable and precise solution. Here are the prominent ways this technology is shaping the future of GPCR research:
True Pharmacology: By enabling the assessment of GPCRs in their native form, it safeguards the integrity of receptor pharmacology, paving the way for data that mirrors the true physiological interactions more closely.
Relevance in Data: The technology allows for the assay of endogenously expressed GPCRs, ensuring that the data generated is highly relevant and reflective of the in-vivo conditions, thus enhancing the accuracy of preliminary screenings in drug development processes.
Expanding Target Pool: MULTISCREENTM Sensor Technology facilitates the characterization of orphan GPCRs, broadening the scope of potential targets and advancing opportunities in GPCR research and drug development.
Accelerated Drug Development: Perhaps one of the most remarkable features is its compatibility with a single cell line for conducting multiple GPCR assays. This not only streamlines the operational workflow but remarkably accelerates the pace of drug development, saving both time and resources while maintaining a high standard of reliability and efficiency.
By prioritizing unmodified GPCRs, MULTISCREENTM Sensor Technology stands as a formidable tool in the researcher’s arsenal, promising a revolution in GPCR-targeted drug development through enhanced efficacy and the true realization of the potentials hidden in GPCR signaling pathways.
MULTISCREEN™ Assay Solutions
Our sensor technology is available as a portfolio of reagents, kits, and services to meet your specific assay needs:
- MULTISCREEN™ reagent options
- HSV and Bacmam viral particles carrying MULTISCREEN™ β-Arrestin sensor genes
- β-Arrestin sensors plasmids
- MULTISCREEN™ Assay Kits (Catalog MSBAK01)
- MULTISCREEN™ BacMam or HSV Assay Kits (Catalog MSBAKBM01-1, MSBAKHSV01-1)
- Cell Lines
- HEK293 and CHO parental cell lines stably expressing MULTISCREEN™ sensors
- 20+ GPCR stable cell lines co-expressing β-Arrestin sensor
- > 1000 GPCR-expressing cell lines
- Services
- Custom stable cell line generation co-expression β-Arrestin sensor and GPCR
- Development of MULTISCREEN™ HTS assays
- GPCR screening and profiling services using β-Arrestin assays
GPCR-Arrestin Co-Expressed MULTISCREENTM Stable Cell lines
| Receptor Family | Receptor | Species | Parental | Stable Cell Lines | Division-Arrested Cells | Membranes |
|---|---|---|---|---|---|---|
| Adrenergic | beta2 | human | HEK293T β-Arrestin1 | CA1438BA1 | DCA1438BA1 | MCA1438BA1 |
| beta2 | human | HEK293T β-Arrestin2 | CA1438BA2 | DCA1438BA2 | MCA1438BA2 | |
| Angiotensin | AT1 | human | HEK293T β-Arrestin1 | HA1417- BA1 | DHA1417- BA1 | MHA1417- BA1 |
| AT1 | human | HEK293T β-Arrestin2 | HA1417-BA2 | DHA1417-BA2 | MHA1417-BA2 | |
| Cannabinoid | CB1 MUTANT B | human | CHO-K1 β-Arrestin2 | CA1513BA2-1 | DCA1513BA2-1 | MCA1513BA2-1 |
| CB2 | human | CHO-K1 β-Arrestin2 | CA1230BA2-1 | DCA1230BA2-1 | MCA1230BA2-1 | |
| CB2 | mouse | CHO-K1 β-Arrestin2 | CAm1230BA2-1 | DCAm1230BA2-1 | MCAm1230BA2-1 | |
| CB2 | rat | CHO-K1 β-Arrestin2 | CAr1230BA2-1 | DCAr1230BA2-1 | MCAr1230BA2-1 | |
| CB2 | rat | CHO-K1 β-Arrestin2 | CAr1230BA2-1a | DCAr1230BA2-1a | MCAr1230BA2-1a | |
| Chemokine | CXCR7 | human | HEK293T β-Arrestin2 | CA1150-BA2 | DCA1150-BA2 | MCA1150-BA2 |
| Citric Acid Cycle Intermediates | GPR91 | human | CHO-K1 B-Arrestin2 | CA1141BA2-1 | DCA1141BA2-1 | MCA1141BA2-1 |
| Free Fatty Acid | GPR120L | human | HEK293T β-Arrestin2 | CA1522 | DCA1522 | MCA1522 |
| GPR40 | human | CHO-K1 β-Arrestin2 | CA1101-1 | DCA1101-1 | MCA1101-1 | |
| GPR40 | rat | CHO-K1 β-Arrestin2 | CAr1101-1 | DCAr1101-1 | MCAr1101-1 | |
| GPR40 | cynomolgus monkey | CHO-K1 β-Arrestin2 | CApc1101-1 | DCApc1101-1 | MCApc1101-1 | |
| Glucagon | GIP | human | HEK293T β-Arrestin2 | CA1290 | DCA1290 | MCA1290 |
| GIP | human | HEK293T β-Arrestin2 | CA1290BA1 | DCA1290BA1 | MCA1290BA1 | |
| GLP-1 | human | CHO-K1 β-Arrestin2 | CA1267-1 | DCA1267-1 | MCA1267-1 | |
| GLP-1 | human | HEK293T β-Arrestin2 | CA1267BA2 | DCA1267BA2 | MCA1267BA2 | |
| Glucagon | human | CHO-K1 β-Arrestin2 | CA1266-1 | DCA1266-1 | MCA1266-1 | |
| Histamine | H4 | human | HEK293T β-Arrestin2 | CA1030BA2 | DCA1030BA2 | MCA1030BA2 |
| Opioid | delta | human | CHO-K1 β-Arrestin2 | CA1351-1 | DCA1351-1 | MCA1351-1 |
| kappa | human | CHO-K1 β-Arrestin2 | CA1352-1a | DCA1352-1a | MCA1352-1a | |
| kappa | human | CHO-K1 β-Arrestin2 | CA1352BA2-1 | DCA1352BA2-1 | MCA1352BA2-1 | |
| mu | human | CHO-K1 β-Arrestin2 | CA1350-1a | DCA1350-1a | MCA1350-1a | |
| NOP | human | CHO-K1 β-Arrestin2 | CA1354-1 | DCA1354-1 | MCA1354-1 | |
| Orphan | GPR35 (short form) | human | CHO-K1 β-Arrestin2 | CA1096-1 | DCA1096-1 | MCA1096-1 |
| GPR35 (long form) | human | CHO-K1 β-Arrestin2 | CA1523-1 | DCA1523-1 | MCA1523-1 | |
| GPR35 (long form) T108M Mutant | human | CHO-K1 β-Arrestin2 | CA1524-1 | DCA1524-1 | MCA1524-1 | |
| GPRC5A | human | HEK293T β-Arrestin2 | CA1525 | DCA1525 | MCA1525 | |
| MRGX2 | human | HEK293T β-Arrestin2 | CA1257aBA2 | DCA1257aBA2 | MCA1257aBA2 | |
| Proton-Sensing Receptors | GPR4 | human | HEK293T β-Arrestin2 | CA1100 | DCA1100 | MCA1100 |
| GPR65 | human | HEK293T β-Arrestin2 | CA1121 | DCA1121 | MCA1121 | |
| GPR68 | human | HEK293T β-Arrestin2 | CA1123 | DCA1123 | MCA1123 | |
| GPR132 | human | HEK293T β-Arrestin2 | CA1066 | DCA1066 | MCA1066 | |
| Parental Cells | HEK293T β-Arrestin2 | CA0001 | MCA0001 | |||
| CHO-K1 β-Arrestin2 | CA0001-1 | MCA0001-1 |
The innovation of MULTISCREEN™ and diverse methods of use brought to the drug discovery research community a new way of assaying signal transduction through β-arrestins without having to tag GPCRs. This high throughput screening technology can be used in MULTISCREEN™ Stable Cell Lines that co-express GPCRs and β-Arrestin, and transiently introduced with BacMam into primary or heterologous cells expressing unmodified GPCRs stably or transiently.
