Receptor-Mediated Endocytosis of G Protein-Coupled Receptors Analyzed by Live Cell Flow Cytometry
Introduction
Background
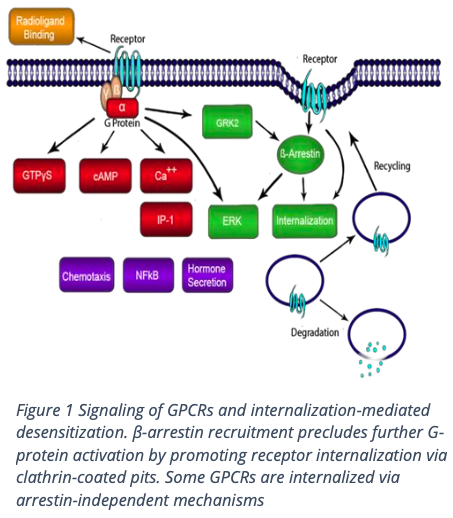
Methodology
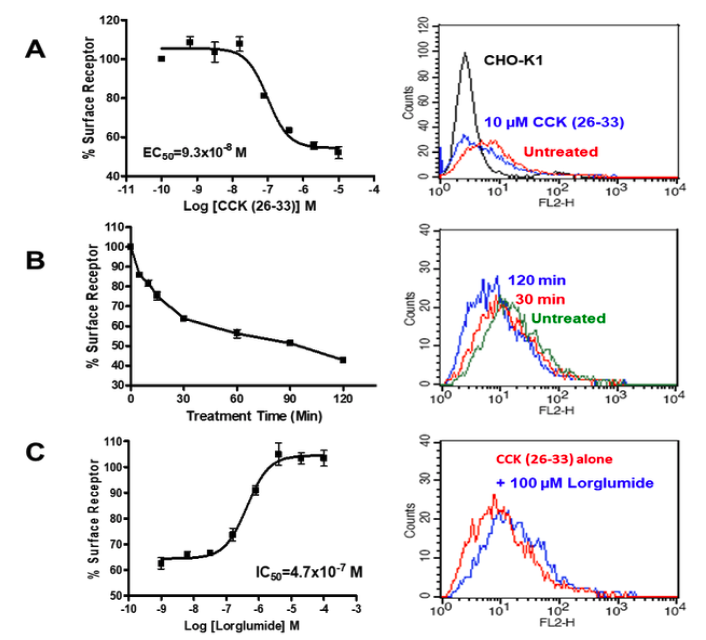
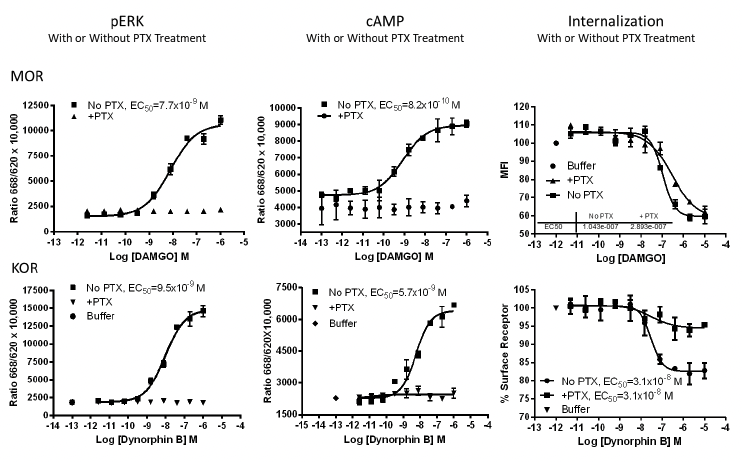
Applications
About the MULTISCREEN™ 231-GPCR Panel
FAQs
References
Moo EV, van Senten JR, Bräuner-Osborne H, Møller TC. Arrestin-dependent and -independent internalization of g protein-coupled receptors: methods, mechanisms, and implications on cell signaling. Mol Pharmacol. 2021;99(4):242-255PMID 33472843
2.Weinberg ZY, Puthenveedu MA. Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis. Traffic. 2019;20(2):121-129PMID 30536564
3.Multispan, Inc | Scientific Insight: 231-GPCR Cell-Based Assay Panel (multispaninc.com)
4.Smith JS, Lefkowitz RJ, Rajagopal S. Biased signalling: from simple switches to allosteric microprocessors. Nat Rev Drug Discov. 2018;17(4):243-260.PMID 29302067
5.Multispan, Inc | Cell-Based Assay Development for GPCRs and Beyond
6.Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of μ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. 2013 Jan;65(1):223–54PMID 23321159
7.Abosamak NER, Shahin MH. Beta 2 Receptor Agonists/Antagonists. [Updated 2022 Jul 4]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-.Available from: https://www.ncbi.nlm.nih.gov/books/NBK559069/
8.Conroy JL, Free RB, Sibley DR. Identification of G protein-biased agonists that fail to recruit β-arrestin or promote internalization of theD1 dopamine receptor. ACS Chem Neurosci. 2015;6(4):681-692PMID 25660762

Recent Comments