Multiscreen 231-GPCR Cell-Based Assay Panel
Download PDF
GPCRs have historically been among the most important classes of pharmaceutical targets across a wide range of clinical indications. Recent clinical, scientific and technical advances have also opened new therapeutic avenues to exploit members of this class. In this paper, we discuss how Multispan’s Multiscreen GPCR panel can provide important insights during the development of new compounds and in repurposing existing therapies.
In this article, we discuss applications of the Multiscreen 231-GPCR cell-based assay panel in drug discovery that exploit recent advances in our understanding of this important class of therapeutic targets [1, 2]. Recent analyses show that drugs targeting GPCRs represent approximately 33% of currently approved drugs and suggest that this class of receptors will continue to represent clinically validated targets across high value indications. The robust screening capabilities enabled by Multiscreen-231™ together with the use of engineered systems for unambiguous target validation and relevant animal disease models will continue to drive improved GPCR drug discovery to meet this need.
Opportunities and challenges in targeting GPCR signaling
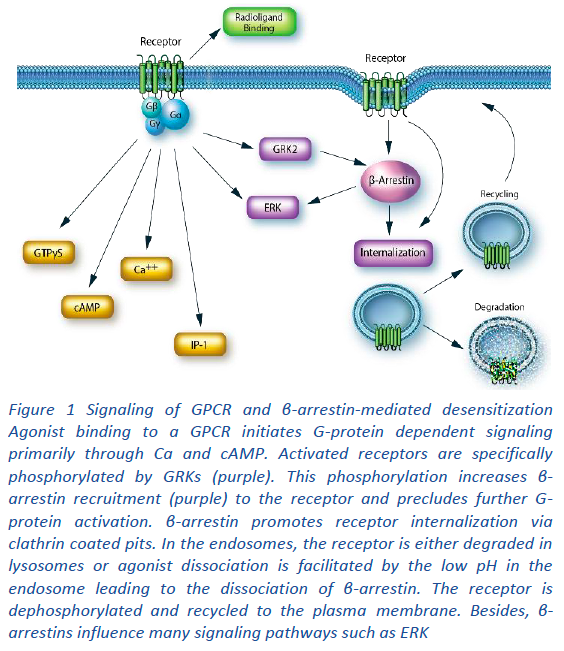
A variety of approaches have evolved to tune activity against GPCRs to fit a specific pharmacological profile [2]. In addition to classical orthosteric antagonists/agonists, GPCR activity can also be modulated agonistically or antagonistically at allosteric sites, opening new avenues for therapeutic intervention [1, 2]. Biased agonism, where different ligands can preferentially activate different intracellular signalling pathways (see Figure 1) opens still further therapeutic opportunities.
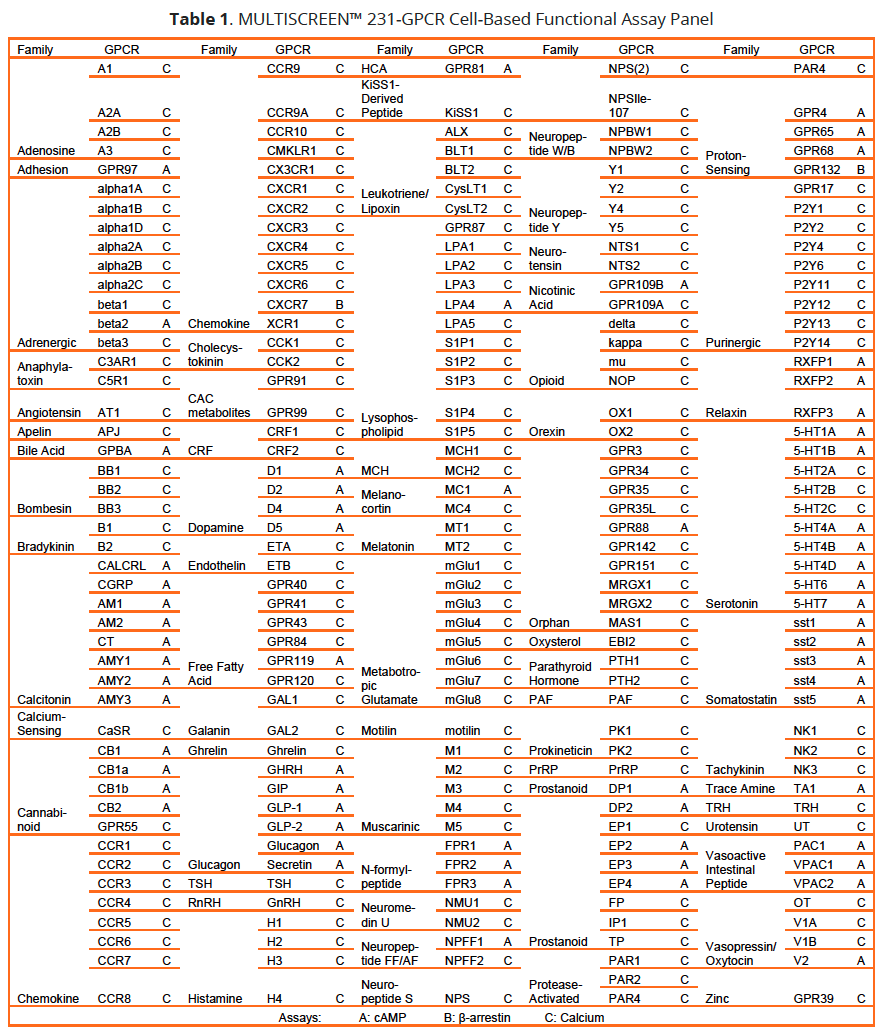
At the same time, this complexity compounds the problem of identifying leads with adequate specificity since the same receptor may be engaged in in multiple interrelated pathways with different downstream effects. [3, 4]. This problem can be addressed by determining the activity of library compounds in both the therapeutically relevant pathway and using counterscreens to assess compound effects on alternate mechanisms of receptor modulation. To address these issues, and to facilitate successful GPCR targeting campaigns, Multispan has developed cell-based assays for a panel of over 231 GPCR targets covering 66 receptor subfamilies (Table 1).
Introducing the MULTISCREENTM231-GPCR Panel
The MULTISCREENTM231-GPCR Panel provides a robust, physiologically relevant and comprehensive solution for high-throughput compound profiling and its versatility makes it amenable to application at all stages of drug development.
A variety of approaches have evolved to tune activity against GPCRs to fit a specific pharmacological profile [2]. In addition to classical orthosteric antagonists/agonists, GPCR activity can also be modulated agonistically or antagonistically at allosteric sites, opening new avenues for therapeutic intervention [1, 2]. Biased agonism, where different ligands can preferentially activate different intracellular signalling pathways (see Figure 1) opens still further therapeutic opportunities.
At the same time, this complexity compounds the problem of identifying leads with adequate specificity since the same receptor may be engaged in in multiple interrelated pathways with different downstream effects. [3, 4]. This problem can be addressed by determining the activity of library compounds in both the therapeutically relevant pathway and using counterscreens to assess compound effects on alternate mechanisms of receptor modulation. To address these issues, and to facilitate successful GPCR targeting campaigns, Multispan has developed cell-based assays for a panel of over 231 GPCR targets covering 66 receptor subfamilies (Table 1).
Introducing the MULTISCREENTM231-GPCR Panel
The MULTISCREENTM231-GPCR Panel provides a robust, physiologically relevant and comprehensive solution for high-throughput compound profiling and its versatility makes it amenable to application at all stages of drug development.
Cell-based assays. The MULTISCREENTM231-GPCR Panel utilizes receptor-dependent, G protein-coupled cellular secondary messenger readouts including calcium and cAMP (Figure 1). Cell-based assays directly capture the specific functional activity of a test compound in a biologically relevant context. They offer clear advantages over historical radioligand binding assays and as such offer an important phenotypic readout to complement data from such direct binding assays between the molecule and target. They can also help elucidate the mechanism of action of the ligand. Gaining these mechanistic readouts upfront, in a cellular context, adds tremendous value in understanding the potential impacts of target modulation as an aid to compound evaluation during lead generation.
to complement data from such direct binding assays between the molecule and target. They can also help elucidate the mechanism of action of the ligand. Gaining these mechanistic readouts upfront, in a cellular context, adds tremendous value in understanding the potential impacts of target modulation as an aid to compound evaluation during lead generation.
Stable cell line clones. To perform reliable assays with a large panel of GPCR targets in high throughput, consistency is key. In the MULTISCREENTM231-GPCR Panel, each GPCR target is expressed in a single, stable clonal cell line that has been carefully selected and rigorously characterized to ensure proper pharmacology with the receptor’s natural agonist. Native ligands are used as internal controls in every compound profiling experiment. Receptor expression levels have been optimized for the functional assays in all these stable clonal cell lines, which are also verified for their stability over multiple passages over a minimum period of 2 months. This ensures consistent expression of each GPCR over time, in contrast to transient transfections, which typically result in highly variable transgene expression levels between runs. Due to their expression consistency, the stable cell lines make it possible to establish, optimize and standardize a robust assay protocol for each GPCR target that can be followed as a reliable SOP every time. Therefore, this expansive set of cell lines collectively serves as a valuable cornerstone of comprehensive compound profiling and drug repurposing efforts, yielding robust and reproducible results.
 to complement data from such direct binding assays between the molecule and target. They can also help elucidate the mechanism of action of the ligand. Gaining these mechanistic readouts upfront, in a cellular context, adds tremendous value in understanding the potential impacts of target modulation as an aid to compound evaluation during lead generation.
to complement data from such direct binding assays between the molecule and target. They can also help elucidate the mechanism of action of the ligand. Gaining these mechanistic readouts upfront, in a cellular context, adds tremendous value in understanding the potential impacts of target modulation as an aid to compound evaluation during lead generation.
Stable cell line clones. To perform reliable assays with a large panel of GPCR targets in high throughput, consistency is key. In the MULTISCREENTM231-GPCR Panel, each GPCR target is expressed in a single, stable clonal cell line that has been carefully selected and rigorously characterized to ensure proper pharmacology with the receptor’s natural agonist. Native ligands are used as internal controls in every compound profiling experiment. Receptor expression levels have been optimized for the functional assays in all these stable clonal cell lines, which are also verified for their stability over multiple passages over a minimum period of 2 months. This ensures consistent expression of each GPCR over time, in contrast to transient transfections, which typically result in highly variable transgene expression levels between runs. Due to their expression consistency, the stable cell lines make it possible to establish, optimize and standardize a robust assay protocol for each GPCR target that can be followed as a reliable SOP every time. Therefore, this expansive set of cell lines collectively serves as a valuable cornerstone of comprehensive compound profiling and drug repurposing efforts, yielding robust and reproducible results.
Applications of the MULTISCREENTM231-GPCR Panel
Compound profiling and Lead generation. Multiscreen-231 can be utilized in a variety of ways depending on the stage and requirements of a compound profiling program.
For example, it might be used to profile new chemotypes against a defined subset of targets, such as the chemokine receptor family, to support lead optimization for a program targeting a specific chemokine receptor. Alternatively, a subset of the full GPCR panel might be used to identify targets for a molecule that has shown efficacy in an in vivo pain model. Rather than profiling the full GPCR panel, in this case it would make sense to run a focused CNS GPCR panel instead.
Additionally, the broad representation of all GPCR subfamilies across the panel maximizes opportunities for target deconvolution of in vivo active compounds, both in preclinical discovery and drug repurposing.
The panel also provides the added benefit of detecting cellular mechanisms of action upfront while addressing potential off-target activities against other GPCRs. The results from these unique and comprehensive compound profiling efforts will therefore enable accelerated timelines to new clinical candidates and uncover novel indications for existing drugs.
Addressing receptor similarity and molecular promiscuity. Multiscreen-231™ can be used to detect undesirable off-target effects of lead candidates in early-stage discovery, potentially enabling structural modifications to improve specificity and safety. It is critical to assess lead candidates early in the discovery process to ensure selectivity for the intended target(s). If they remain uncharacterized, off-target activities could lead to harmful adverse effects in clinical trials and contribute to costly drug development failures.
The challenge presented by GPCRs is their ubiquitous expression in human tissues, and their essential physiological roles that impact both pathogenesis and health. Furthermore, receptors in the same subfamilies and beyond frequently share high similarity, and some known endogenous ligands can functionally activate multiple GPCRs. Many of the drugs that currently target GPCRs are small molecules and synthetic peptides, and many of these also exhibit molecular promiscuity and polypharmacology and inadvertently engage other GPCRs that were not intended as drug targets. Recognizing this, a variety of approaches have evolved to tune activity against GPCRs to fit a specific pharmacological profile. These include allosteric modulators, biased ligands, targeting of oligomeric GPCR forms, and manipulation of polypharmacology of either small molecules, peptides, proteins or antibodies. At the core of these approaches is the use of a robust and comprehensive assay panel that can specifically quantify cross-reactivity against a range of GPCRs, covering all the different subfamilies to provide critical data to aid in compound evaluation.
Evaluation of drugs with known function, but unknown mode-of-action. Multiscreen-231™ represents a valuable toolkit to facilitate accelerated discovery of novel targets for purified active compounds with previously unknown mode-of-action (MoA). The target and mode of action of many therapeutic agents or bioactive compounds (such as the herbal remedies used in traditional Chinese medicine (TCM)) remain unknown. Identifying the active species, drug targets and molecular mechanisms of TCM preparations in order to understand and characterize their MoA is of significant therapeutic interest. MoA determination is a key step in realizing the benefits of these valuable traditional medicines through the regulated clinical drug development process, [5]. Because of the inherent druggability of GPCRs and their omnipresence in regulatory processes affecting all physiological functions including CNS, cardiovascular, inflammation, metabolism and cell growth, they are an important protein class in the quest for TCM target deconvolution.
As an assay panel that broadly covers GPCRs from different families, each with a distinct and well-defined biological function, Multiscreen-231 can facilitate successful target identification and MoA elucidation. These studies can open possibilities for ‘leap-frogging’ to much-needed new therapeutic solutions in areas of unmet need, such as non-addictive pain killers, [6] or oncology drugs for solid tumors that are resistant to chemotherapy and existing immunoregulatory agents. [7]
Repurposing of marketed drugs. More than 130 GPCRs are targeted by approved drugs, and it is estimated that ∼700 such drugs modulate at least one GPCR target. (8) Drug repurposing refers to the strategy of identifying new uses, or secondary indications, for approved or investigational drugs that lie outside the scope of the original indication(s). [9] Drug repurposing is highly attractive due to its shortened preclinical and clinical development timeline which reduce overall research and development costs. As a result, drug repurposing is an attractive proposition in GPCR drug discovery because of the large number of existing drugs against this target class.
As mentioned above, the binding promiscuity of small molecules and proteins that can lead to side effects can also be seen as an opportunity for drug repurposing or defined polypharmacological strategies. Target identification and MoA elucidation therefore have renewed importance in evaluating drug use in other disease settings and against alternative targets [10,11]. Furthermore, the repurposing principle not only applies to approved GPCR drugs, but also to marketed drugs directed against other target classes. This approach therefore expands the utility of the large, heterogeneous repertoire of chemical and biological therapeutics with established clinical safety, by uncovering their novel and potentially useful effects on different GPCR targets [8, 9]. Multispan-231 therefore fit directly into screens to support drug repurposing studies.
Compound profiling and Lead generation. Multiscreen-231 can be utilized in a variety of ways depending on the stage and requirements of a compound profiling program.
For example, it might be used to profile new chemotypes against a defined subset of targets, such as the chemokine receptor family, to support lead optimization for a program targeting a specific chemokine receptor. Alternatively, a subset of the full GPCR panel might be used to identify targets for a molecule that has shown efficacy in an in vivo pain model. Rather than profiling the full GPCR panel, in this case it would make sense to run a focused CNS GPCR panel instead.
Additionally, the broad representation of all GPCR subfamilies across the panel maximizes opportunities for target deconvolution of in vivo active compounds, both in preclinical discovery and drug repurposing.
The panel also provides the added benefit of detecting cellular mechanisms of action upfront while addressing potential off-target activities against other GPCRs. The results from these unique and comprehensive compound profiling efforts will therefore enable accelerated timelines to new clinical candidates and uncover novel indications for existing drugs.
Addressing receptor similarity and molecular promiscuity. Multiscreen-231™ can be used to detect undesirable off-target effects of lead candidates in early-stage discovery, potentially enabling structural modifications to improve specificity and safety. It is critical to assess lead candidates early in the discovery process to ensure selectivity for the intended target(s). If they remain uncharacterized, off-target activities could lead to harmful adverse effects in clinical trials and contribute to costly drug development failures.
The challenge presented by GPCRs is their ubiquitous expression in human tissues, and their essential physiological roles that impact both pathogenesis and health. Furthermore, receptors in the same subfamilies and beyond frequently share high similarity, and some known endogenous ligands can functionally activate multiple GPCRs. Many of the drugs that currently target GPCRs are small molecules and synthetic peptides, and many of these also exhibit molecular promiscuity and polypharmacology and inadvertently engage other GPCRs that were not intended as drug targets. Recognizing this, a variety of approaches have evolved to tune activity against GPCRs to fit a specific pharmacological profile. These include allosteric modulators, biased ligands, targeting of oligomeric GPCR forms, and manipulation of polypharmacology of either small molecules, peptides, proteins or antibodies. At the core of these approaches is the use of a robust and comprehensive assay panel that can specifically quantify cross-reactivity against a range of GPCRs, covering all the different subfamilies to provide critical data to aid in compound evaluation.
Evaluation of drugs with known function, but unknown mode-of-action. Multiscreen-231™ represents a valuable toolkit to facilitate accelerated discovery of novel targets for purified active compounds with previously unknown mode-of-action (MoA). The target and mode of action of many therapeutic agents or bioactive compounds (such as the herbal remedies used in traditional Chinese medicine (TCM)) remain unknown. Identifying the active species, drug targets and molecular mechanisms of TCM preparations in order to understand and characterize their MoA is of significant therapeutic interest. MoA determination is a key step in realizing the benefits of these valuable traditional medicines through the regulated clinical drug development process, [5]. Because of the inherent druggability of GPCRs and their omnipresence in regulatory processes affecting all physiological functions including CNS, cardiovascular, inflammation, metabolism and cell growth, they are an important protein class in the quest for TCM target deconvolution.
As an assay panel that broadly covers GPCRs from different families, each with a distinct and well-defined biological function, Multiscreen-231 can facilitate successful target identification and MoA elucidation. These studies can open possibilities for ‘leap-frogging’ to much-needed new therapeutic solutions in areas of unmet need, such as non-addictive pain killers, [6] or oncology drugs for solid tumors that are resistant to chemotherapy and existing immunoregulatory agents. [7]
Repurposing of marketed drugs. More than 130 GPCRs are targeted by approved drugs, and it is estimated that ∼700 such drugs modulate at least one GPCR target. (8) Drug repurposing refers to the strategy of identifying new uses, or secondary indications, for approved or investigational drugs that lie outside the scope of the original indication(s). [9] Drug repurposing is highly attractive due to its shortened preclinical and clinical development timeline which reduce overall research and development costs. As a result, drug repurposing is an attractive proposition in GPCR drug discovery because of the large number of existing drugs against this target class.
As mentioned above, the binding promiscuity of small molecules and proteins that can lead to side effects can also be seen as an opportunity for drug repurposing or defined polypharmacological strategies. Target identification and MoA elucidation therefore have renewed importance in evaluating drug use in other disease settings and against alternative targets [10,11]. Furthermore, the repurposing principle not only applies to approved GPCR drugs, but also to marketed drugs directed against other target classes. This approach therefore expands the utility of the large, heterogeneous repertoire of chemical and biological therapeutics with established clinical safety, by uncovering their novel and potentially useful effects on different GPCR targets [8, 9]. Multispan-231 therefore fit directly into screens to support drug repurposing studies.
References
- Cooke RM, Brown AJH, Marshall FH, Mason JS. Structures of G protein-coupled receptors reveal new opportunities for drug discovery. Drug Discovery Today. 2015;20(11). doi:10.1016/j.drudis.2015.08.003
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: New agents, targets and indications. Nature Reviews Drug Discovery. Published online 2017. doi:10.1038/nrd.2017.178
- Christopoulos A, Changeux JP, Catterall WA, et al. International union of basic and clinical pharmacology. XC. Multisite pharmacology: Recommendations for the nomenclature of receptor allosterism and allosteric ligands. Pharmacological Reviews. 2014;66(4). doi:10.1124/pr.114.008862
- Devree BT, Mahoney JP, Vélez-Ruiz GA, et al. Allosteric coupling from G protein to the agonist-binding pocket in GPCRs. Nature. 2016;535(7610). doi:10.1038/nature18324
- Zhang WJ, Huai Y, Miao ZP, Qian AR, Wang YH. REVIEW article: Pharmacology Systems for Investigation of the Mechanisms of Action of Traditional Chinese Medicine in Drug Discovery. Front. Pharmacol., 2019; doi.org/10.3389/fphar.2019.00743
- Alhassen L, Dabbous T, Ha A, Dang LHL, Civelli O The Analgesic Properties of Corydalis yanhusuo. Review Molecules 2021; 10;26(24):7498. doi: 10.3390/molecules26247498. PMID: 34946576 PMCID: PMC8704877
- Liu SH, Chen PS, Huang CC, Hung YT, Lee MY, Lin WH, \ Lin YC, Lee AYL. PERSPECTIVE article: Unlocking the Mystery of the Therapeutic Effects of Chinese Medicine on Cancer. Front. Pharmacol., 2021; doi.org/10.3389/fphar.2020.601785
- Pushpakom S, Iorio F, Eyers PA, et al. Drug repurposing: Progress, challenges and recommendations. Nature Reviews Drug Discovery. Published online 2018. doi:10.1038/nrd.2018.168
- Cha Y, Erez T, Reynolds IJ, et al. Drug repurposing from the perspective of pharmaceutical companies. British Journal of Pharmacology. 2018;175(2). doi:10.1111/bph.13798
- Sriram K, Insel PA. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Molecular Pharmacology. 2018;93(4):251-258. doi:10.1124/mol.117.111062
- Chartier M, Morency LP, Zylber MI, Najmanovich RJ. Large-scale detection of drug off-targets: Hypotheses for drug repurposing and understanding side-effects. BMC Pharmacology and Toxicology. 2017;18(1). doi:10.1186/s40360-017-0128-7b
Download PDF

Recent Comments