Hit to Lead and Lead Optimization in Drug Discovery:
In Vitro Pharmacology Support
Where to Start with Hit to Lead (H2L) Drug Discovery Research
The process of early drug discovery starts with deciding which disease to address and finding a druggable target for that disease and validating that target. Following validation, a high throughput in vitro pharmacology assay is developed to identify target activity, and a high-throughput screen (HTS) of a small molecule chemical compound library is typically performed. The size of the library can vary between tens of thousands to a few million compounds. In the HTS assay, several hits will be identified.
Following confirmation of the hit identification in the primary assay, these molecules may then be binned into different chemical series, and decisions can then be made on which series to chemically optimize into molecules that are more amenable to being developed into pharmaceutically optimized lead compounds and eventually a potential drug.
The Hit to Lead Process
Hit to lead is an iterative process of lead improvement. It is the stage where a hit, typically identified in a high throughput screen, is chemically modified into a promising lead molecule following improvements in activity against the target. This stage, part of the discovery phase of drug development, requires an intimate association between medicinal chemists building the molecules and biologists performing the primary screen. This process also includes creating a structure activity relationship (SAR) for a better understanding of pharmacological activity resulting from the chemical structure.
It is critical that the screen is well validated, i.e. reproducible and depicts the in vitro pharmacology activity to be optimized, and that the compound activity data are quickly returned to chemists to ensure that chemical modifications can be performed in a rapid and timely manner. In addition, it is important that any assay-related questions can be addressed immediately.
At Multispan, we have developed both the ability to develop and run well-validated cell-based GPCR assays and the ability to return data to the client in a single-day turnaround. Control tool compound is run with every plate whose activity is tracked and shared along with each set of raw and analyzed compound data. This ensures and documents assay consistency and quality from week-to-week throughout the entire duration of the campaign.
Assay with Confidence
GPCRs have multiple readouts indicating different pathways of activity, and compounds can be biased against one pathway or the other. We typically work with our clients to identify the appropriate GPCR disease-relevant isoform that is physiologically relevant to the GPCR level of expression and correct pathway. For example, for partial agonists, we have found that GPCR expression level is critical to success—too high a level and partial agonists appear as full agonists, while too low a level results in agonists without any activity. We have built custom cell lines to exhibit the appropriate physiological activity.
Efficacy-gap Reduction between Partial Agonist and Full Agonists in Dose-Response Curve cAMP Assay in D1-HEK293 Stable Clones. Correlation with Increased D1-Receptor Surface Expression in the D1-HEK293 Stable Clones Measured by FACS.
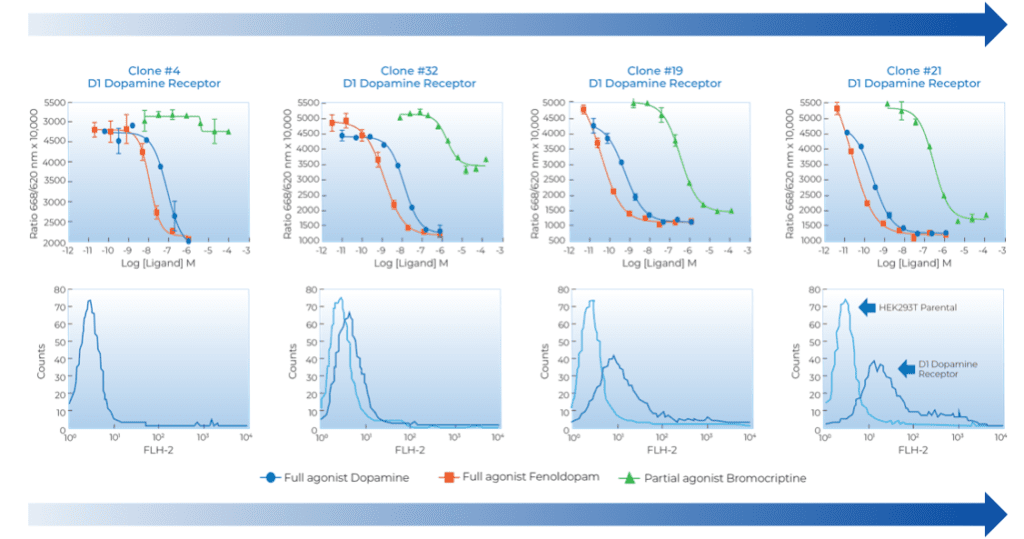
Figure. Example of How Surface Expression Impacts Dopamine Receptor D1 Pharmacology in Multispan’s Stable Cell Line Clones
With full collaboration with our clients, we jointly identify the correct conditions to run the screen and perform follow-up studies. We also can run assays in parallel, if required, such as in 2 different signaling pathways.
Hit to Lead Services and Lead Optimization
Once a lead has been identified, additional optimization is required to make the molecule more pharmacologically acceptable or more “drug-like”.
These preclinical candidates are further developed to improve their properties. These modifications can be to improve chemical properties such as solubility and protein binding as well as drug-like properties that can predict success in vivo. Drug-like properties include cell permeability (MDCK), hERG activity, PGP activity, cyp inhibition, to name a few. Along this process, the primary target activity must be followed and improved, and activity against off-target GPCRs must be tested.
At Multispan, we work closely with clients to provide the follow-up primary activity assay as well as assays for closely related GPCRs. In addition, we check the activity of the drug candidate compounds against the orthologs of species in which the in vivo activity will be measured. This is a critical step to ensure that, although we are developing drugs for humans, we can also test them in vivo in animal models to predict human activity. These in vitro assays are provided at Multispan See Figure 2, article MULTISCREEN™ Stable Cell Lines.
Finally, off-target activity against GPCR panels should be tested. These off targets can predict known physiological issues. At Multispan, we have developed a panel of 32-GPCRs that, if inhibited, have known pharmacological problems. This panel can be run with a 2-week turnaround. If a different panel is requested, such as all CNS-related GPCRs, the panel can be developed and tested, also with equally rapid turnaround.
Full Solution
With all of the resources that Multispan can offer, we can enable your GPCR drug discovery to better free up your hands to perform other tasks such as target validation. With our expertise, we can provide rapid assay development, validation, testing and secondary testing to move your molecules forward toward an IND efficiently.
Download Article PDF

Recent Comments