High Throughput Screening Done Right
The Power of Integrating Screening and Assay Development
Discovering new drugs requires a careful balance of speed and quality. Appropriate screens must be developed to measure a molecule’s ability to alter a target’s biological activity. Hence, assay consistency and quick result delivery is of the utmost importance for finding new patient treatments.
Assays Configuration
Assays can be configured as either biochemical or cell-based and can be designed with different throughputs.
- High throughput:
- measures the activity of hundreds of thousands of molecules in rapid succession.
- ultimate goal is to find a suitable candidate for a hit to lead program.
- Moderate throughput:
- tests a smaller focused library of up to 100,000 or for a hit to lead program.
A hit to lead program test molecules with similar structure, sourced either by purchase or by chemical modifications of the hit molecular series. This testing reliably measures the difference in potency (EC50) of molecules to drive the structure activity relationships (SAR) and guide chemistry in its molecular modifications. Speed and data quality is of the essence as chemists rely on the answers to improve modifications of the chemical hits.
How Good Must the Assays Be?
For the best results, assays must consistently perform with a z’ of > 0.5. It is recommended that this validation is from a plate of half positive controls and half negative controls.
Key parameters to look for:
- The concentration of positive control should be run at about 80% of its 100% activity (EC80).
- Assays must demonstrate that EC50s are reproducible, i.e. within a factor of 3.
- EC50s from compounds of varying activity should be similar and demonstrate an R2 of > 0.7 when directly compared to each other and run between days.
- A positive control should be run with each assay and must show adequate reproducibility.
What type of assays can be used for GPCRs?
There are a number of ways to measure GPCR activity including membrane assays using radiolabel, such as ligand binding and GTPγS binding, and multiple cell-based assays. These include detection of second messengers such as Ca++, cAMP and inositol phosphate and arrestin translocation, ERK phosphorylation, and downstream cell signaling.
There are advantages for each assay type. It is widely accepted that measuring the most proximal response to the receptor yields a better likelihood for the compounds to affect the receptor directly. However, the most critical piece for each assay is the quality of the reagents; the receptor containing cells are the lynchpin.
Developing Quality Cells and Cell-Based Assays at Multispan.
For over 16 years, Multispan has taken pride in developing the highest quality cell lines and assays. We start by identifying the molecular target and the appropriate parental cell line. We then develop receptor transfected stable cell lines with the appropriate functional response for the target. Receptors can signal via multiple means and exhibit cell signaling bias, so it is critical to use the same cell when measuring all the signals known to stem from a receptor. This common background allows an unambiguous tracking of a compound’s effect on alternate pathways.
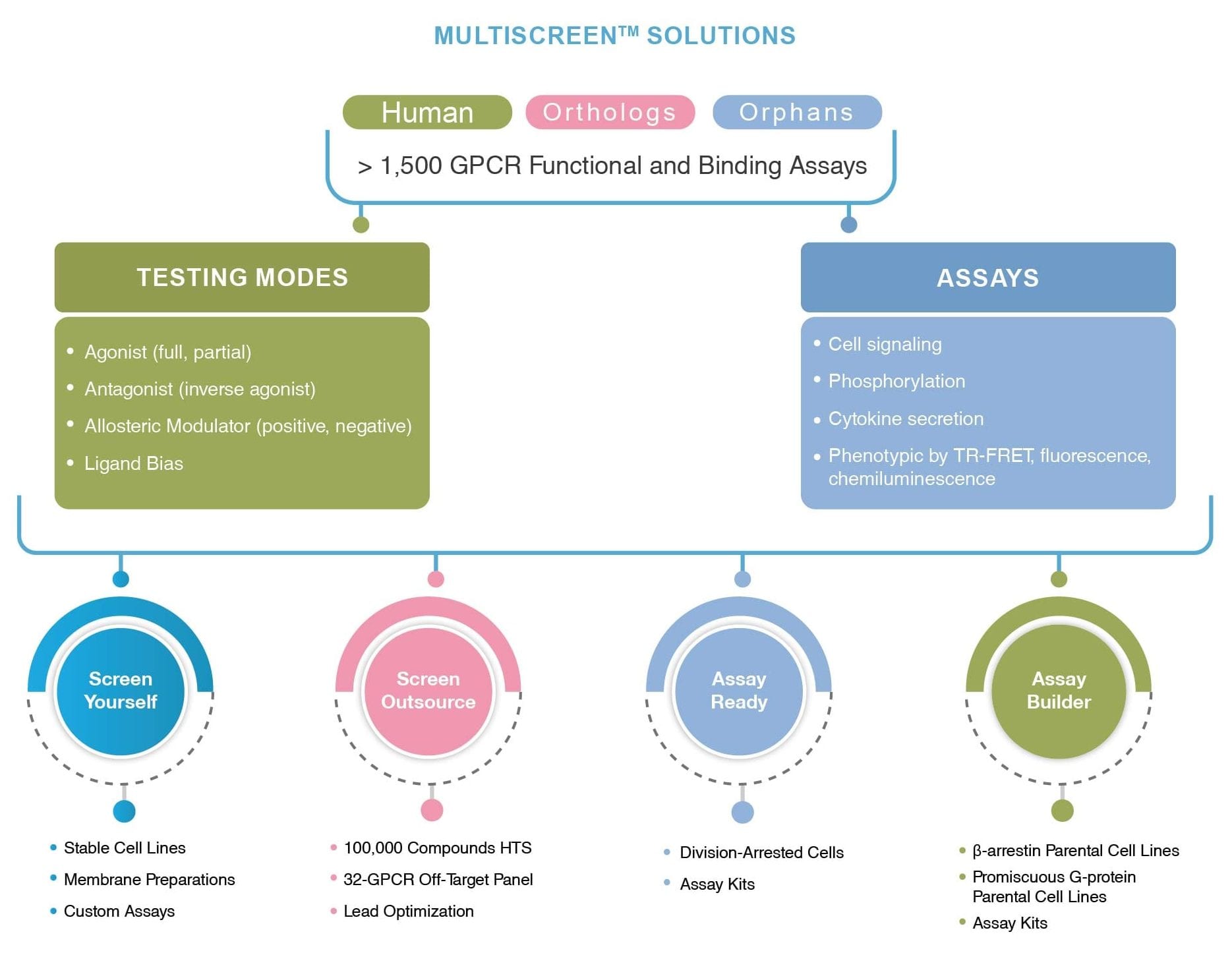
We understand, however, that clients may not have the facilities to maintain cell lines, so we can scale up these cells and ship to the client on an as-needed basis. In cases where the need for cells may be smaller, we have division arrested cells that are validated for all assays and can be run in small screens without the need to grow cells.
If your target is not one that we currently have at Multispan, we can save you time by building your cell line with its assays. We will ensure you stay informed during cell line and assay development, with routine progress checks and opportunities for feedback.
High Throughput Screening Capabilities
With our in vitro pharmacology expertise, we help accelerate drug discovery research by providing comprehensive HTS solutions. We are dedicated to helping customers succeed in every project we take on. Our assays are typically set up as follows:
- Screening compound libraries in 384-well formats with various detection technologies (absorbance, fluorescence, luminescence)
- Cell-based, phenotypic and binding assays formats using detection kits commercially available from Multispan and others, or using custom-assembled reagents to achieve the best results.
Our modalities include:
-
- Calcium
- cAMP
- β-arrestin
- Chemotaxis
- Internalization
- Insulin secretion
- IP-1
- Lucifersae reporter gene assay
- pERK
- pGRK-2
- pNFκB
- Radioligand binding
- GTPγS
- Consultation on assay design, development, optimization, validation and method documentation
- Agonist, antagonist, PAM and NAM modes
- Proprietary technologies and extensive experience guarantee success
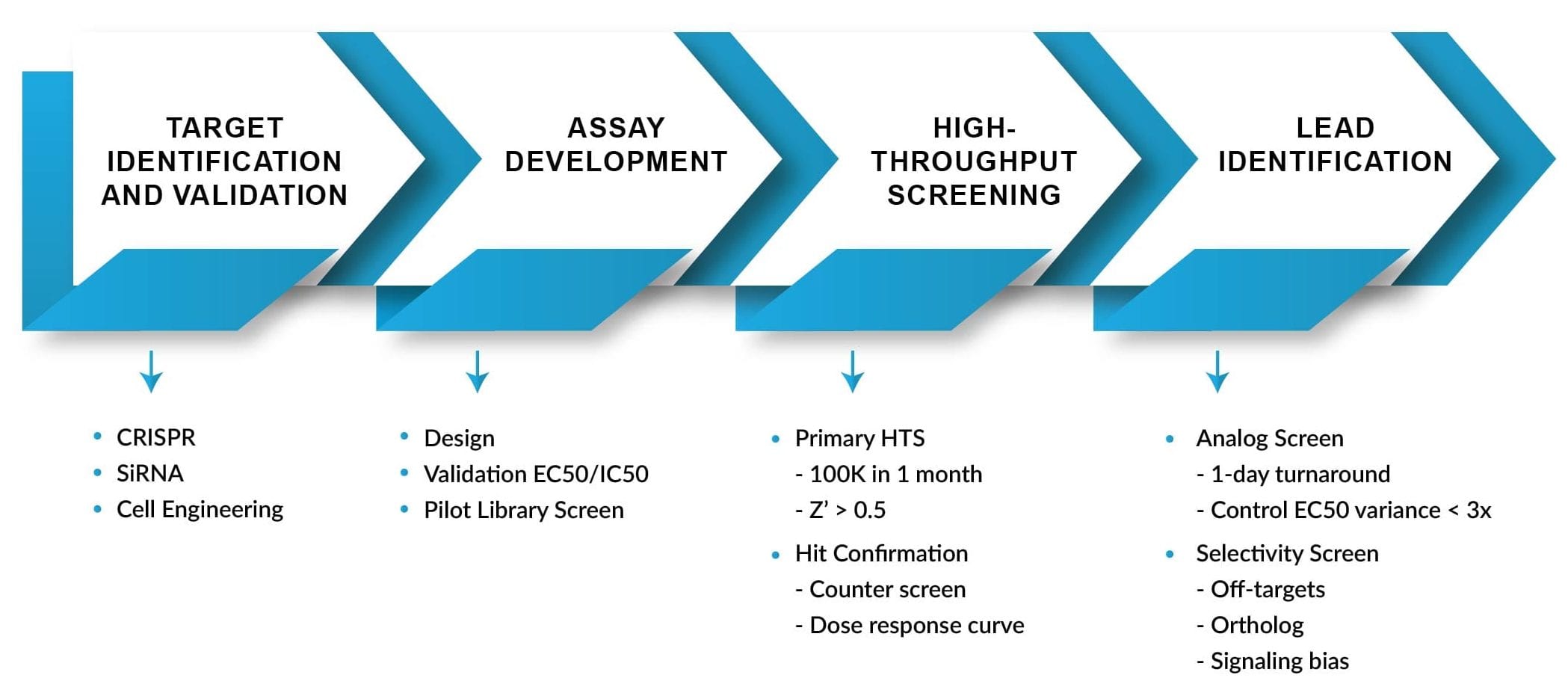
Our Turnaround Time
- Assays for agonist/antagonist/partial agonist, and positive or negative allosteric modulators ⇒ in 3 months
- HTS assay of 100,000 compounds, screening & hit confirmation ⇒ in 1 month
- SAR or lead optimization primary assay ⇒ in 1 day
- SAR or lead optimization secondary assays ⇒ in 1-5 days
Conducting HTS at Multispan with in-house built assays
also saves months wasted on assay transfer!
All screens must demonstrate a suitable z’ and are always run with a positive control. In the rare occasion where a control fails QC, the assay is repeated at our cost. Since we have cell lines for multiple receptor families, including orthologs, we can cross evaluate the activity of hits against these receptors on a routine basis. We also offer a functional safety assessment GPCR panel.
We are a one-stop shop for high throughput screening and secondary compound profiling.
Track Record: Case Study
Our track record is 100% repeat client business.
In an initial evaluation with a now long-term client, our skills were put in competition against 2 other providers. In Phase I, our mission was to test two stable cell lines expressing a human receptor with high and low expression. After the client determined that Multispan had outperformed the competition in data quality, turnaround time and price, we were chosen for a long-term contract.
Phase II was a Lead Optimization project, in which we ran controlled primary screens weekly with next day delivery of data, along with secondary screens for orthologs, radioligand binding and cell signaling bias. A gap in the screening funnel was filled through our custom cell lines and assays, and this project continues to run after 2 years.
Summary
Multispan is a solely US based company, with an established history of providing consistent, well-validated, quality cell lines and assays, for over sixteen years. This is exemplified by our 100% customer return rate. Although our focus has been GPCRs, we also have experience in cell assays for kinases and other native cells, including some CRISPR knockout technologies. Our assay platform includes our own proprietary assays for cAMP, Ca and Arrestin sensors, along with other assay formats. Our turnaround times are second to none, with a 3-month window for novel cell line production and assay development, 1 month for screening and hit validation of 100,000 compounds, and 1 day for routine SAR.
All assays have internal QC and must meet our predetermined specifications, or are repeated at our charge. Our customer satisfaction reflects our dedication to quality, as described by customer testimonials on our web site. If you are looking for unparalleled excellence, competitive pricing and rapid turnaround time in a US-based company, we are your company. We will continue to expand our portfolio and provide quality service for many years to come.


Recent Comments