Exploring The Role of Cyclic AMP in Cellular Signaling for Drug Discovery Research
Understanding the intricacies of intracellular signal transduction mechanisms is pivotal in the quest for developing novel therapeutics. Among the myriad of signaling molecules, cyclic AMP (cAMP) stands out as a quintessential second messenger involved in a plethora of physiological processes. Instead of direct actions, cAMP operates through a nuanced mechanism, as a crucial intermediary in a variety of biological signaling pathways.
General Information
What is Cyclic AMP?
cAMP is a cyclic nucleotide that stands for 3′,5′-cyclic adenosine monophosphate (amp). It is generated from adenosine triphosphate (ATP) by the action of the enzyme adenylyl cyclase. cAMP derives its name from its cyclic structure and consists of an adenosine molecule bonded to a ribose sugar, which is connected to a single phosphate group.
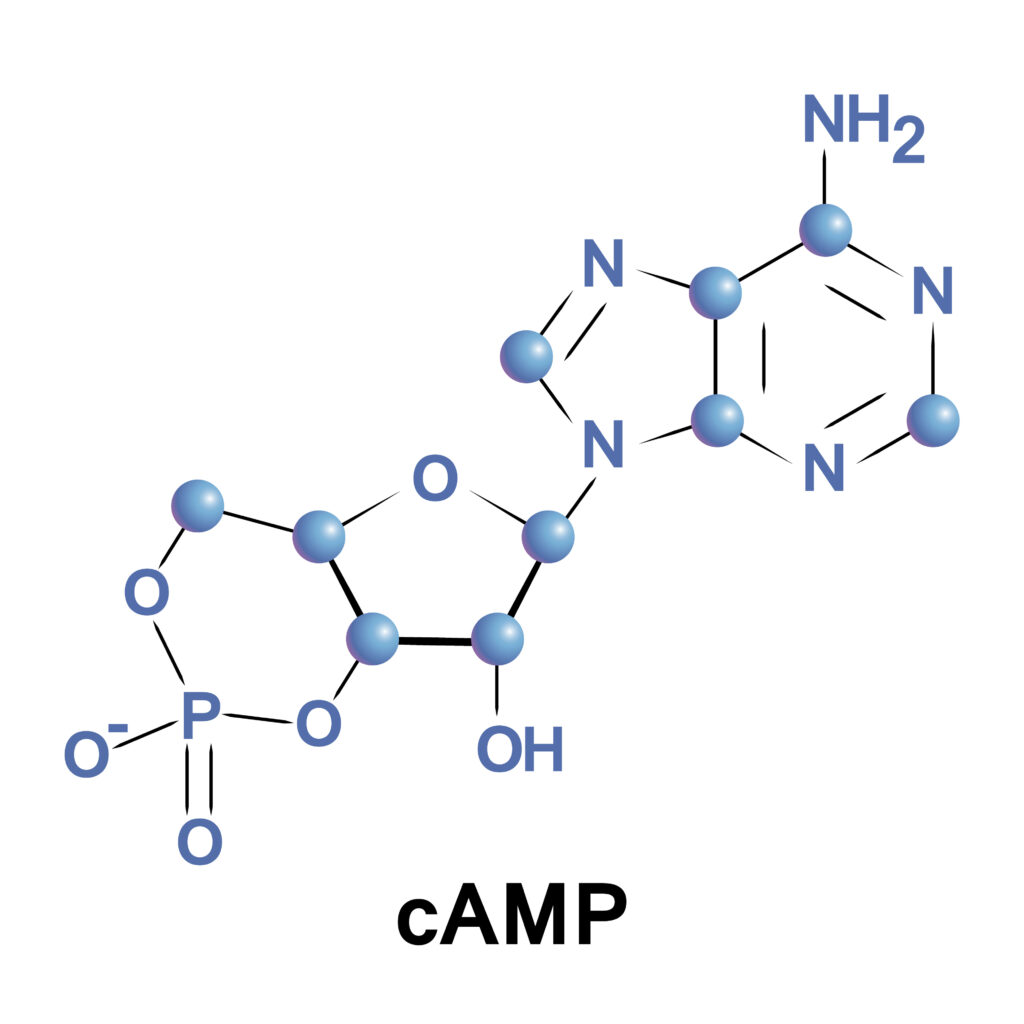
What Does Cyclic AMP Do?
Cyclic AMP function includes the regulation of various physiological responses, including metabolism, gene transcription factors, and cell proliferation.
Cyclic AMP as a Second Messenger
cAMP is a second messenger involved in the transduction of extracellular signals received by cell surface receptors to the interior of the cell. Acting downstream of the initial receptor-ligand interaction, cAMP amplifies the signal within the cell, facilitating a robust physiological response to a relatively minute initial stimulus. This amplification mechanism is integral to the cellular response, making the understanding of cAMP dynamics crucial for drug discovery.
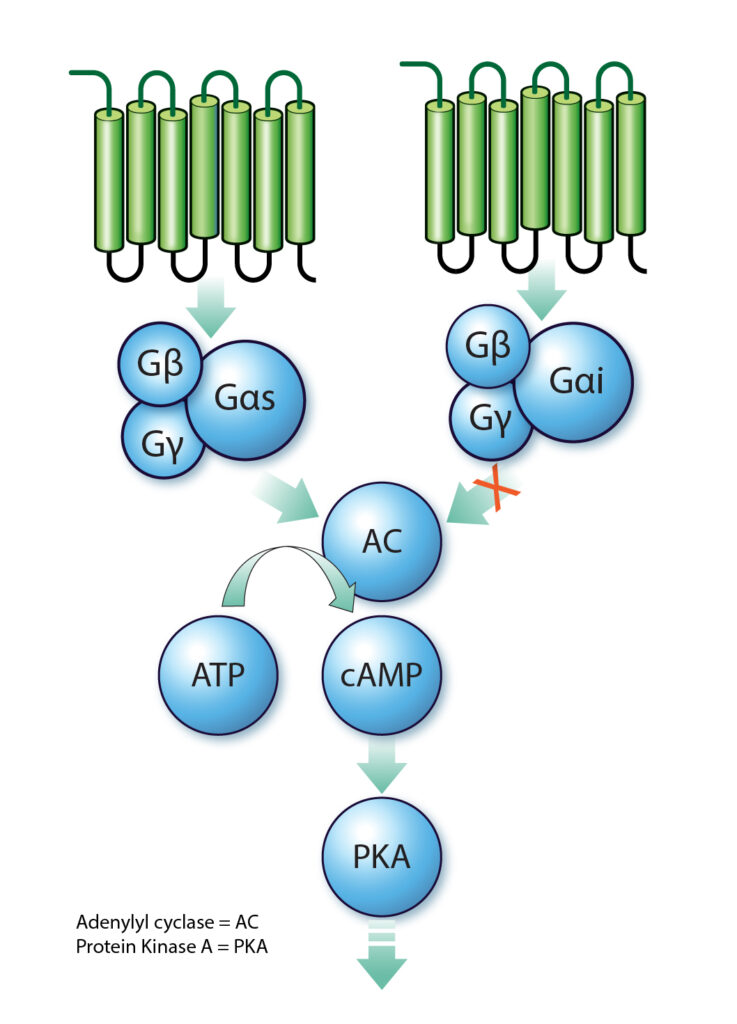
Pathway of Cyclic AMP and GPCR
cAMP exerts its effects by activating protein kinase A (PKA), which in turn phosphorylates various target proteins within the cell. This activation leads to alterations in cell function, contingent upon the specific proteins phosphorylated. The cAMP pathway can be initiated upon the binding of an extracellular ligand to a G protein-coupled receptor (GPCR), culminating in the activation or inhibition of adenylyl cyclase and the subsequent production or decrease of cAMP level inside the cell.
Adenylyl cyclase is usually stimulated by the Gαs subunit (which promotes cAMP production), but inhibited Gαi subunit, both are released through activation of their respective coupled GPCR. This cascade underscores the versatility of cAMP as a mediator of diverse signaling pathways.
Implications for Drug Discovery
The central role of the cAMP pathway in numerous signaling networks makes it an attractive target for therapeutic intervention. Drugs that modulate the production or degradation of cAMP and its upstream signaling like GPCRs have the potential to treat a wide range of diseases, from metabolic disorders to cancer. For instance, targeting GPCR-mediated the cAMP pathway can influence heart rate and force of contraction in heart disease or modulate insulin release in diabetes.
Despite its therapeutic potential, targeting the cAMP pathway presents unique challenges. The ubiquitous nature of cAMP signaling necessitates precise modulation to avoid unintended consequences. However, this also opens avenues for novel therapeutic approaches. For example, selective activation or inhibition of specific adenylyl cyclase isoforms could provide targeted therapeutic effects with minimal side effects. Furthermore, understanding the interaction of cAMP with other signaling molecules, such as cyclic GMP, could lead to the development of drugs with dual actions, potentially offering more effective treatments with fewer side effects.
Areas of Focus

Drugs targeting G protein-coupled receptors (GPCRs)
Many of these drugs transmit signals through cAMP-mediated pathways. By understanding the intricacies of cAMP signaling, researchers can design drugs that specifically target GPCRs associated with various diseases. For example, beta-adrenergic receptors, involved in cardiovascular regulation, are known to modulate cAMP levels. Drugs that selectively modulate these receptors can have implications for conditions like heart failure and hypertension.

Phosphodiesterases (PDEs)
Phosphodiesterases (PDEs), enzymes responsible for cAMP degradation, are another critical target in drug discovery. Inhibitors of PDEs can elevate intracellular cAMP levels, influencing downstream signaling events. Compounds targeting specific PDE isoforms have shown promise in the treatment of conditions such as chronic obstructive pulmonary disease (COPD) and erectile dysfunction. For instance, phosphodiesterase type 5 (PDE5) inhibitors, like sildenafil (Viagra), elevate cAMP levels and are used in the management of erectile dysfunction.

Protein Kinase A (PKA)
Furthermore, cAMP technology contributes to the understanding and development of drugs targeting the cAMP-dependent protein kinase A (PKA) pathway. PKA is a key effector downstream of cAMP, and modulating its activity can impact various cellular processes including the regulation of ion channels. This pathway is implicated in cancer, and researchers are exploring PKA modulators as potential anticancer agents. By targeting specific components of the cAMP/PKA pathway, researchers aim to develop drugs with enhanced efficacy and reduced side effects compared to traditional chemotherapy.
The modulation of cAMP levels, whether through GPCRs, PDEs, or the PKA pathway, offers a rich landscape for the development of therapeutics targeting a wide range of diseases.
Detecting Intracellular Cyclic AMP
Measuring cAMP levels is crucial in studying various cellular processes and signaling pathways. There are several methods available for assaying intracellular cAMP levels.
Radioimmunoassay (RIA)
RIA is a traditional method that uses radiolabeled cAMP and a specific cAMP antibody. The binding of the antibody to cAMP is measured through radioactivity, providing a quantitative measure of intracellular cAMP levels. However, the use of radioisotopes raises safety concerns.
Enzyme Immunoassay (EIA) or Enzyme-Linked Immunosorbent Assay (ELISA)
ELISA-assay uses antibodies to detect and quantify cAMP using an enzyme to produce a measurable signal such as fluorescence. This and the several methods described below are isotope-free and amenable to high-throughput screening.
Fluorescence Resonance Energy Transfer (FRET) Sensors
FRET-based cAMP sensors use fluorescent proteins to detect changes in cAMP levels. These sensors consist of two fluorophores, and changes in cAMP alter the distance between them, resulting in a change in FRET signal. This method allows real-time monitoring of intracellular cAMP dynamics.
In addition to the above commonly used cAMP assays, Bioluminescence Resonance Energy Transfer (BRET), cAMP-Glo™, Immunocytochemistry and Imaging, and High-Performance Liquid Chromatography (HPLC) are a few other options.
When selecting a method for measuring intracellular cAMP, consider factors such as sensitivity, specificity, ease of use, and the compatibility of the method with your experimental system.
MULTISCREEN™ TR-FRET cAMP No Wash Assay
The MULTISCREEN™ TR-FRET cAMP 1.0 No Wash Assay, along with MULTISCREEN™ GPCR stable cell lines and division-arrested cells, and optimized assay protocols bring unparalleled 3-in-1 solution to experimental setups. With these cross-validated complete MULTISCREEN™ solutions, Multispan empowers researchers in compound screening.
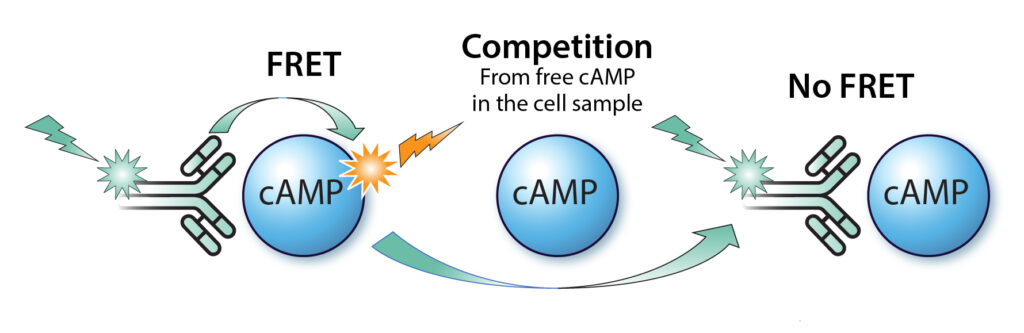
Our MULTISCREEN™ assay kit employes TR-FRET (Time-Resolved Fluorescence Resonance Energy Transfer) as the readout. It offers a homogeneous assay method using a dual-label system, MULTISCREEN™ Eu-labeled cAMP antibody (Ab) and MULTISCREEN™ 650-labeled cAMP, which competes with intracellular free cAMP in binding to the cAMP labeled antibody. The distinct fluorescence lifetimes of the labels enable the precise determination of cAMP concentration that can be read by most commercially available multimode microplate readers.
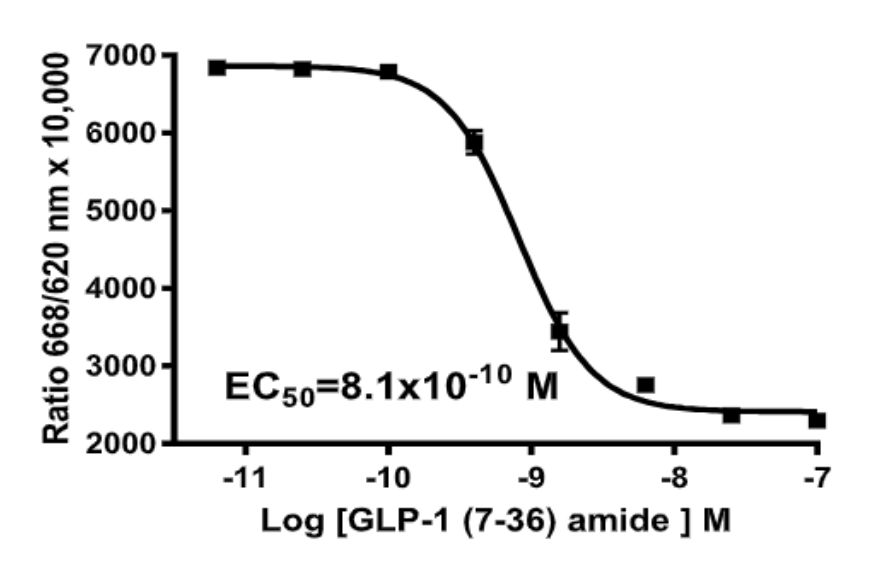
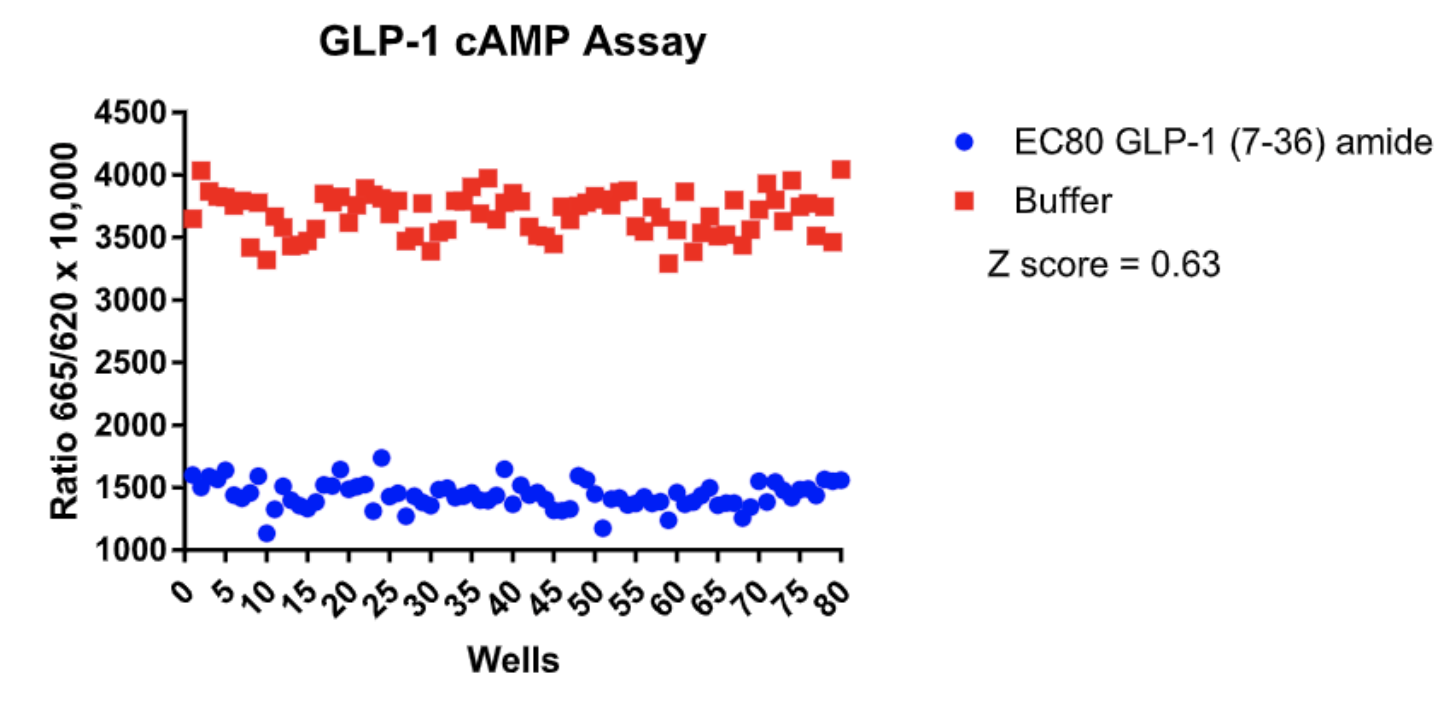
Left Figure: cAMP Dose-Response in MULTISCREEN™ GLP-1 β-Arrestin2 HEK293T stable cell line and measured with MULTISCREEN™ TR-FRET cAMP 1.0 No Wash Assay Kit. Right Figure: Representative Z’-factor data from one data set conducted with MULTISCREEN™ GLP-1 β- Arrestin2 HEK293T stable cell line measured with MULTISCREEN™ TR-FRET cAMP 1.0 No Wash Assay Kit on a BioCoat Poly-D-Lysine 384-well plate in the absence (red) or presence of agonist (blue).
Advantages of MULTISCREEN™ TR-FRET cAMP No Wash Assay
Like the other MULTISCREEN™ kits, MULTISCREEN™ TR-FRET cAMP 1.0 No Wash Assay Kit is carefully designed for high throughput screening (HTS) assay purpose which also undergoes stringent validation by MULTISCREEN™ HTS-ready stable cell lines and division-arrested cells. Here are a few distinct advantages of this assay kit.
High-Throughput Miniaturization
MULTISCREEN™ GPCR Assay Kits excel in highly miniaturized settings, operating effectively in 96-well, 384-well, or higher density microplates.
Comprehensive Solutions
MULTISCREEN™ stable cell lines and division-arrested cells synergize seamlessly with MULTISCREEN™ assay kits, creating a holistic solution for your drug discovery needs.
Detailed Assay Protocols
Our comprehensive protocols guide you from cell preparation to assay execution, enhancing the reliability and consistency of your results.
Proven Validity
Routine validation and utilization of our assays in compound screening campaigns by Multispan’s laboratory ensure the reliability of your experimental outcomes.
Expert-Driven Solutions
Developed and supported by our team of cell and assay experts, our kits empower you with expert-level capabilities for successful cell biology research and drug discovery compound screening.
Efficient Workflow
This NO-WASH HOMOGENEOUS assay eliminates unnecessary steps, ensuring a smooth and efficient workflow. This key feature is consistent across all MULTISCREEN™ assay kits, guaranteeing streamlined processes without compromising data quality.



