-
Assays & Services
MULTISCREENTM Products
Services
Innovations
Have any Questions or Want a Quote?
Talk to a SpecialistSign Up For Our Newsletter
Email Sign Up -
Support
-
Assays & Services
MULTISCREENTM Products
Services
Innovations
Have any Questions or Want a Quote?
Talk to a SpecialistSign Up For Our Newsletter
Email Sign Up -
Support
Artificial Intelligence in Biotechnology
Exploring AI in the Context of Drug Discovery Research
Drug discovery remains a critical endeavor in the development of modern therapeutics for many reasons: the threat of life expectancy from chronic conditions like mental health and metabolic diseases, the emergence of new infections endangers global health, and greater than 500 common and 10,000 rare diseases without treatment, to name just a few.
To urgently revolutionize the drug discovery methodology to achieve efficiency, sustainability and scale serves to provide answers to the treatment of modern health concerns.
With breakthroughs in computer systems technology, namely artificial intelligence, is there an intersection with cutting-edge biotechnology research where artificial intelligence be leveraged to accelerate the drug discovery process?
How Does Generative AI Accelerate the Early Stages of Drug Discovery?


The drug discovery process traditionally begins by identifying a target molecule implicated in a disease’s pathology through cellular mechanisms, literature review, and database exploration. However, the rapid growth of biological data from high-throughput screening, omics technologies poses challenges in data management and analysis. Artificial intelligence (AI) addresses these challenges by quickly analyzing vast datasets from diverse sources like clinical trials and scinetific journals, providing a holistic approach to target identification.
Machine learning, a subset of AI, excels at uncovering patterns and relationships in complex datasets, potentially revealing novel drug targets by identifying associations between genetic markers, proteins, and disease pathology.
After identifying potential drug targets, validating their role in the disease becomes crucial, but it’s often costly and time-consuming due to extensive experimental work, including in vitro and in vivo studies. Traditional assays and models without nuanced biology considerations may fail to accurately mimic human disease conditions, leading to false conclusions and wasting resources. Validation can also be slow, especially when dealing with numerous targets, causing bottlenecks. Additionally, demonstrating that observed effects stem from the intended target rather than off-target effects is vital.
Leveraging AI, particularly machine learning and deep learning algorithms, enables predictive analysis of target modulation effects, prioritizing targets with the highest therapeutic potential and reducing false outcomes. AI-driven simulations and computational models can swiftly test thousands of scenarios, significantly expediting the target-validation process.
After validating promising targets, the focus shifts to finding potential drug molecules, or “hits,” that interact effectively with the target through compound screening. This traditionally involves testing large chemical libraries, often consisting of greater than 1 million chemical compounds, using high-throughput screening (HTS), to identify active compounds. This is a time consuming and resource-intensive step including human and plastics.
Whereas AI models, especially generative AI models can swiftly analyze existing data from all existing databases, accelerating de novo novel hit generation with much reduced resource requirements.
Once hits are identified, they undergo lead optimization to enhance therapeutic potency, selectivity, and safety. Machine learning models enhance this process by predicting how new compounds might interact with the target, allowing for prioritization of compounds with greater therapeutic potential.
Early assessment of a compound’s Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) properties is crucial for determining drug suitability. AI models, trained on historical data, can predict safety issues and ADMET profiles, aiding in the early elimination of unsuitable candidates during screening.
The drug discovery process traditionally begins by identifying a target molecule implicated in a disease’s pathology through cellular mechanisms, literature review, and database exploration. However, the rapid growth of biological data from high-throughput screening, omics technologies poses challenges in data management and analysis. Artificial intelligence (AI) addresses these challenges by quickly analyzing vast datasets from diverse sources like clinical trials and scinetific journals, providing a holistic approach to target identification.
Machine learning, a subset of AI, excels at uncovering patterns and relationships in complex datasets, potentially revealing novel drug targets by identifying associations between genetic markers, proteins, and disease pathology.
After identifying potential drug targets, validating their role in the disease becomes crucial, but it’s often costly and time-consuming due to extensive experimental work, including in vitro and in vivo studies. Traditional assays and models without nuanced biology considerations may fail to accurately mimic human disease conditions, leading to false conclusions and wasting resources. Validation can also be slow, especially when dealing with numerous targets, causing bottlenecks. Additionally, demonstrating that observed effects stem from the intended target rather than off-target effects is vital.
Leveraging AI, particularly machine learning and deep learning algorithms, enables predictive analysis of target modulation effects, prioritizing targets with the highest therapeutic potential and reducing false outcomes. AI-driven simulations and computational models can swiftly test thousands of scenarios, significantly expediting the target-validation process.
After validating promising targets, the focus shifts to finding potential drug molecules, or “hits,” that interact effectively with the target through compound screening. This traditionally involves testing large chemical libraries, often consisting of greater than 1 million chemical compounds, using high-throughput screening (HTS), to identify active compounds. This is a time consuming and resource-intensive step including human and plastics.
Whereas AI models, especially generative AI models can swiftly analyze existing data from all existing databases, accelerating de novo novel hit generation with much reduced resource requirements.
Once hits are identified, they undergo lead optimization to enhance therapeutic potency, selectivity, and safety. Machine learning models enhance this process by predicting how new compounds might interact with the target, allowing for prioritization of compounds with greater therapeutic potential.
Early assessment of a compound’s Absorption, Distribution, Metabolism, Excretion, and Toxicity (ADMET) properties is crucial for determining drug suitability. AI models, trained on historical data, can predict safety issues and ADMET profiles, aiding in the early elimination of unsuitable candidates during screening.

How Can Multispan Empower Your AI Drug Discovery Endeavors?
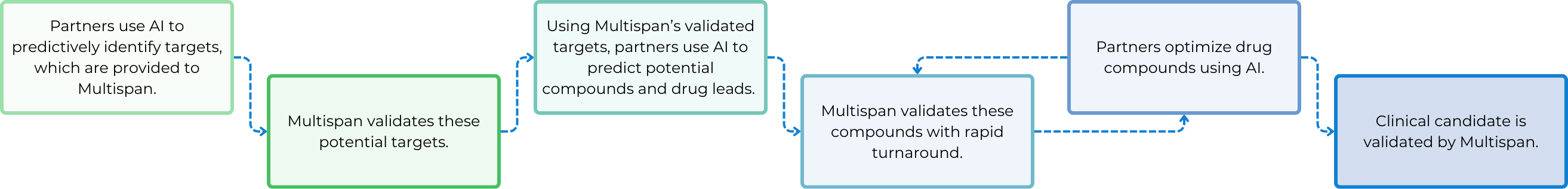
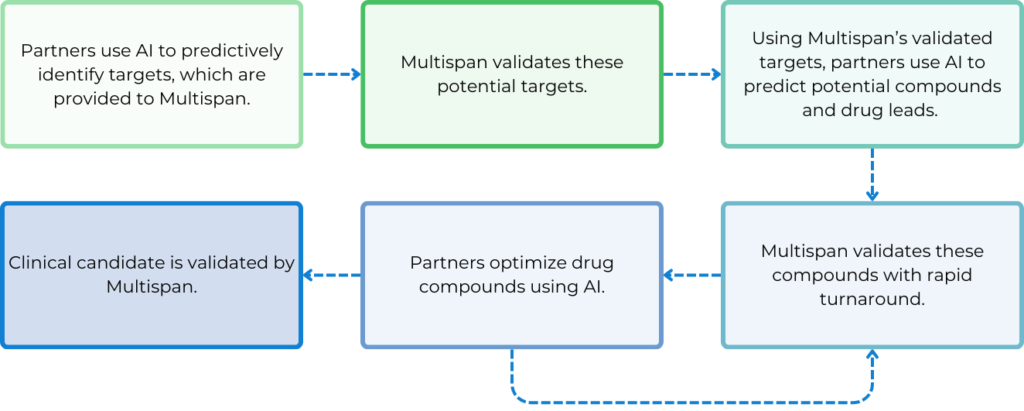
The Key Role of Cell-Based Assays for AI Drug Discovery
To leverage AI in the transformation of the next-generation drug discovery technologies, the use of biologically relevant cell-based assays to generate high-quality data with fast turnaround is crucial. Identifying disease-relevant cell lines to use and pathways to measure as readouts is essential.
Additionally, pinpointing specific pathogenic target isoforms is vital for each specific program. Besides, developing assays that maximize assay window, consistency, and reliability is also key, along with incorporating counter screen assays to eliminate false positives.
After a screening cascade or funnel is established upfront, it is iteratively utilized throughout target validation, compound screening, hit identification, hit-to-lead, and lead optimization stages. The quality and consistency of assay results can provide real-time feedback to AI algorithms, enabling fast and more accurate predictions of molecules with more and more improved efficacy, potency, specificity and safety. Faster data generation enhances the power of AI and machine learning, making the drug discovery process more efficient and sustainable with increased success rates at each step.
