Meeting the Need for Accelerated COVID Antiviral and Vaccine Development
The rapid emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 has substantially impacted both human health and the global economy. This has highlighted the importance of rapid development of frontline therapeutics to curb viral infection, treat secondary symptoms, and decrease mortality rates. Timelines for de novo drug development are generally measured in years, making this an impractical approach for near-term treatment of emerging pathogens. Thus, researchers look to repurpose approved drugs or triage candidates that have shown success treating similar infectious agents with established safety profiles.
While frontline therapies are instrumental for fighting widespread infection, their efficacy and safety profiles are likely to need improvement to ultimately win the battle against SARS-CoV-2. Developing second-generation therapeutics and protective vaccines requires an increased understanding of the underlying biology of disease and pathogen life cycle as well as more typical drug development hit identification and optimization initiatives.
The creation of cell-based models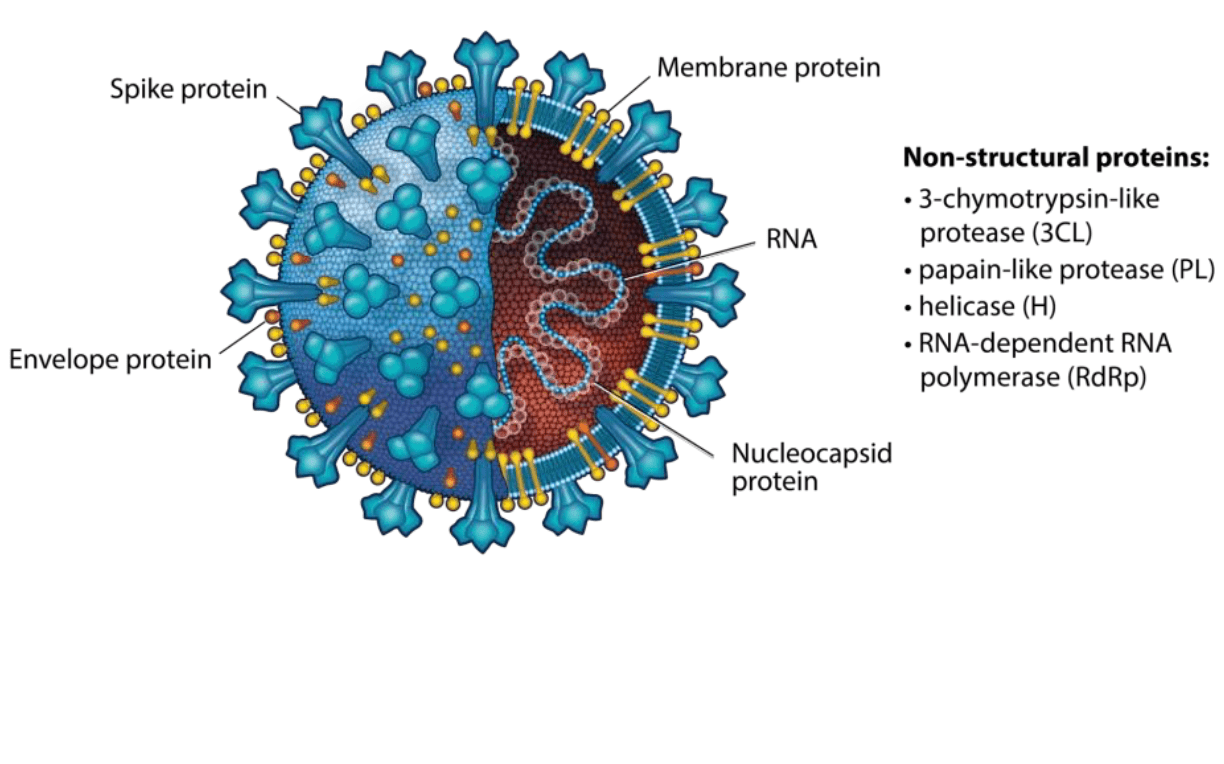
Rapid Repurposing
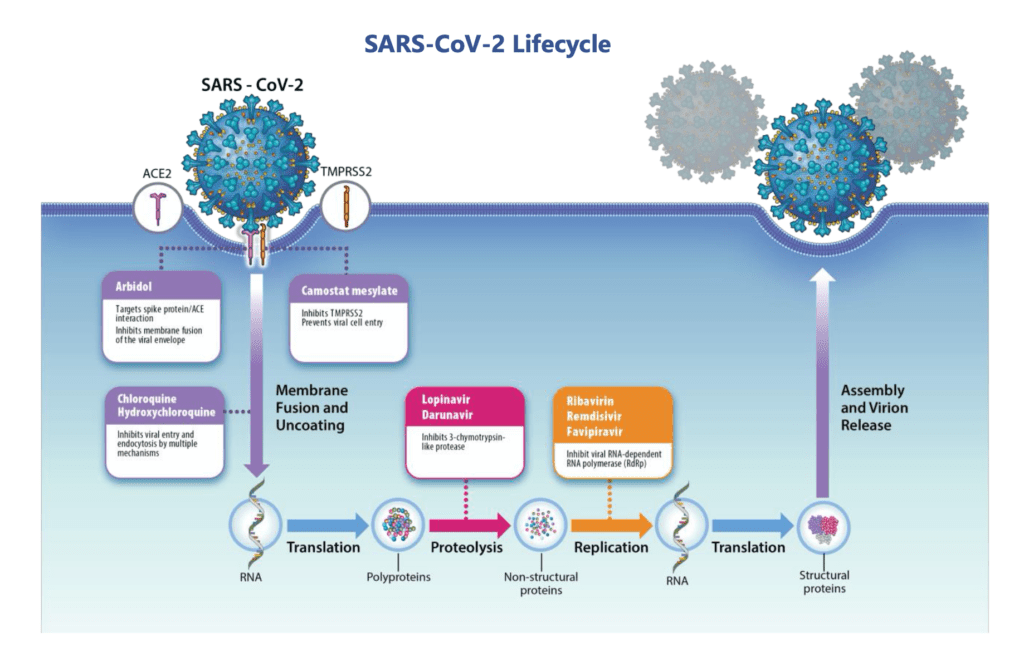
Remdesivir, a small molecule nucleoside analog developed by Gilead Sciences, is a known inhibitor of a range of RNA viruses including Ebola and other Coronaviruses such as those responsible for the severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) outbreaks. Remdesivir acts by interfering with RNA-dependent RNA polymerase (RdRp), an enzyme key to viral replication.
During the West African Ebola virus epidemic remdesivir was clinically evaluated, shown to have a positive safety profile, and was used for near-term control of epidemic spread. Ultimately, however, the drug was shelved in favor of other monoclonal antibody-based treatments which were found to be significantly more effective.1
Early during the emergence of SARS-CoV-2, remdesivir was evaluated and found to block in vitro viral replication and was quickly transitioned to the clinic where it was shown to accelerate patient recovery from 15 to 11 days.2,3 It has received Emergency Use Authorization (EUA) but is generally not thought to be the magic bullet needed to halt the COVID pandemic nor curtail future outbreaks.
Many researchers are now turning to medicinal chemistry and structure activity relationship studies of remdesivir and other known RdRp inhibitors to rationally design a ‘second-generation’ RdRp inhibitor with improved efficacy against SARS-CoV-2.
Remdesivir repurposing has, in fact, been taken one step further. Clinical trials are now underway that look to use it in combination with a second repurposed drug, baricitinab, a Janus kinase (JAK) inhibitor approved for the treatment of rheumatoid arthritis that may help curtail the deleterious cytokine storms seen in severe cases of COVID-19.4
Cell-based Assay: RdRp Inhibitor Screening
Enzymatic activity assays, such as viral polymerase assays, can be performed using cell-free biochemical assay formats. Unfortunately, candidates identified using biochemical assays often do not translate to in vivo efficacy. Cell-based enzymatic assays provide a more physiologically relevant model that results in improved data quality and in vivo translation. To rapidly screen compounds for inhibitory activity against RdRp, cells can be engineered to stably express and assemble a functional SARS-CoV-2 RdRp and a reporter gene can only be transcribed by RdRp to yield reporter protein activity. Polymerase inhibitory activity and potency can readily be assessed in a high throughout format using these engineered cells.
Repurposing Beyond Remdesivir
Although remdesivir has received much of the media coverage about anti-viral drug development, many other drugs are being repurposed and rapidly evaluated as treatments against SARS-CoV-2 — several of which target host or viral proteases.5
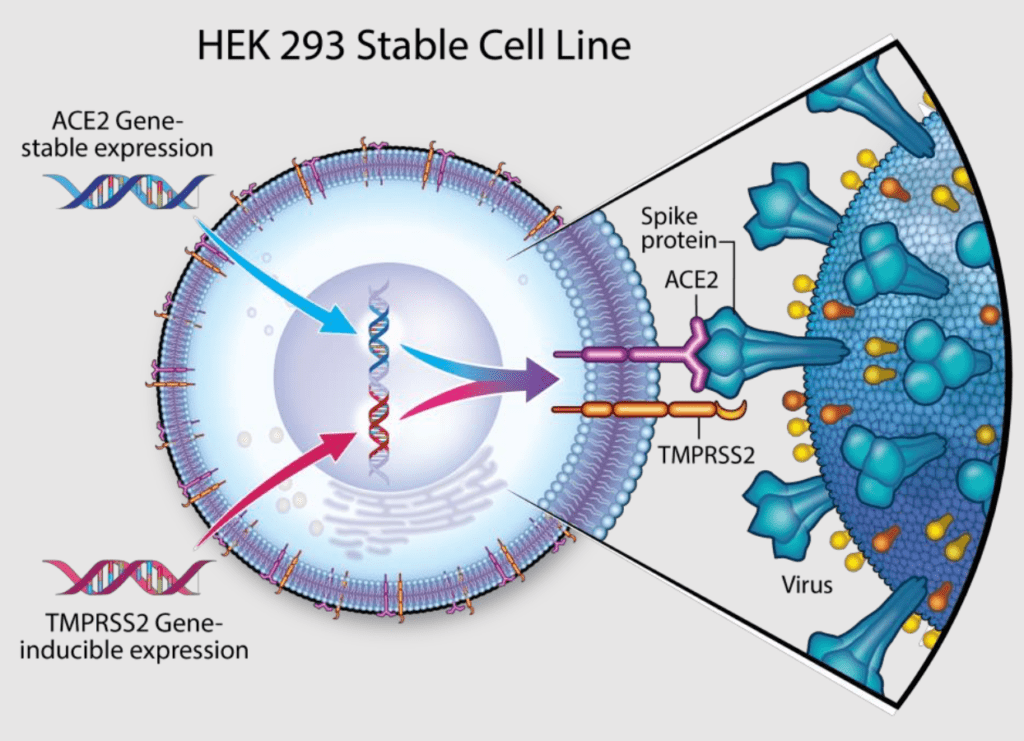
Transmembrane protease serine 2 (TMPRSS2) is a host cell serine protease that is required for cleavage of the viral trimeric spike (S) protein and viral entry. Nafamostat mesylate and Camostat mesylate, known inhibitors of TMPRSS2, are currently approved for treatment of a variety of conditions. Early studies indicate that Camostat can inhibit SARS-CoV-2 entry and have supported initiation of Phase I/II clinical trials.6
SARS-CoV-2 encodes two viral polyproteins that must be cleaved and processed into mature viral proteins. Two SARS-CoV-2 proteases, 3-chymotrypsin-like protease (3CLpro) and papain-like protease (PLpro), are required for polyprotein cleavage and represent high value therapeutic targets.
Because of the high sequence similarity between SARS-CoV-2 and other CoV, previously characterized inhibitors of 3CLpro and PLpro are available for repurposing. Computational approaches, such as machine learning, supported by in vitro cell-based assay confirmation enable rapid repurposing and secondary optimization of these compounds.
Cell-based Assay: Protease
Large numbers of CoV viral protease inhibitors have been characterized. The engineering of quality cell clones co-expressing active viral proteases and reporter protein substrates enable high throughput scrutiny of this large population of potential SARS-CoV-2 protease inhibitors.
Blocking SARS From the Start
One pathway commonly targeted by antivirals, including HIV and other CoV, is viral binding and entry. Frequently this is mediated by antibodies or other molecules that inhibit the interactions needed between host receptor(s) and viral surface proteins.
Similar to SARS-CoV, the SARS-CoV-2 S protein uses angiotensin-converting enzyme 2 (ACE2) as the host cell receptor and requires processing by TMPRSS2 prior to ACE2 binding. Antibodies targeting the SARS-CoV S protein, sera from convalescing SARS-CoV-2 patients, and clinically-proven TMPRSS2 inhibitors have all shown to hinder viral entry illustrating the critical nature, and thus therapeutic relevance, of these protein:protein interactions.7
Many groups are targeting these interactions using antibodies and small molecules as a means to inhibit infection. An alternative approach to reduce viral entry that is currently being evaluated in clinical trials is to therapeutically administer soluble ACE2 to patients.8
Cell-based Assay: Viral Entry
In April of 2020 Hoffmann et al. reported studies that demonstrated SARS-CoV-2 infection depends on S protein interactions with host cell proteins ACE2 and the proteolytic activity of TMPRSS2.7 These studies used a cell-based pseudo virus infectivity assay. One example of such an assay relies on recombinant expression of ACE2 in HEK cells (ACE2- cells) as well as inducible overexpression of TMPRSS2.
Lessons Learned From HIV
In approximately 2 decades HIV diagnosis evolved from a death sentence to a manageable condition. In 1996, the life expectancy of a 20-year-old person with HIV was 39 years. In 2011, the life expectancy improved to approximately 70 years, and in 2020 HIV infection is on the verge of becoming ‘curable’.9
During those years, the scientific community learned an enormous amount about RNA virus pathophysiology, how to therapeutically target these viruses, and how to develop an effective drug regimen that provides sustained anti-viral activity.
Several key lessons –
Use a cocktail of drugs
Target multiple independent viral pathways or enzymes
Continue to develop drugs with incremental improvements in potency, efficacy, and safety profiles
The current multi-drug HIV combination, known as highly active antiretroviral therapy (HAART), targets several viral processes and enzymes. These regimens significantly lower viral loads, sometimes to undetectable levels, eliminate development of drug resistance, maintain the health of patients’ immune systems, and can even prevent transmission. Their continued evolution has increased effectiveness, reduced side effects, improved medication adherence, and made infection with HIV a livable disease.
Similar to HIV, it is likely that an optimum SARS-Cov-2 antiviral treatment with sustained efficacy will contain a combination of drugs that each target different areas of the virus lifecycle. Repurposing efforts are underway that target a number of SARS-CoV2 enzymes and viral processes including the 3Clpro and PLpro proteases and RdRp, offering hope that antiviral cocktails may become a reality and that with continued optimization they can curtail future SARS-CoV-2 outbreaks even in the absence of a protective vaccine.
Helping Make a Difference
Control and eradication of SARS-Cov-2 will require advanced antivirals and/or a protective vaccine – development of both of which depends on improved knowledge of the virus pathophysiology, identification and therapeutic exploitation of the vulnerabilities of the virus, and characterization of the components of protective immunity. As we have learned from other emerging diseases, the solution is likely to be a combination of approaches that take iterative development to optimize efficacy and safety.
A common thread to all these efforts is physiologically-relevant models such as cell-based assays. A variety of assays are needed ranging from general infectivity to highly specific viral pathways including binding, entry, enzymatic activity, maturation, and packaging.
Despite the urgency of the COVID-19 pandemic care must be taken in implementing these assays. They are the foundation of hit identification, lead optimization and early toxicity studies. And thus, the quality– robustness, reproducibility, sensitivity, and specificity — of the assays is key.
Multispan has a proven record of complex cell engineering and assay development. Ensure the advancement of your SARS-CoV-2 drug and vaccine development efforts by harnessing the knowledge and expertise of their scientific teams. Contact Multispan today to discuss the design and implementation of your COVID assay.
References:
1. Mulangu, et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. (2019) N. Engl. J. Med., 381(24):2293-2303.
2. Wang, et.al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. (2020) Cell Res., 30:269-71.
3. Beigel, et al. Remdesivir for the Treatment of Covid-19 – Preliminary Report. (2020) N. Engl. J. Med., 2020 May 22:NEJMoa2007764. doi: 10.1056/NEJMoa2007764. Online ahead of print.
4. Adaptive COVID-19 Treatment Trial 2 (ACTT-II):https://clinicaltrials.gov/ct2/show/NCT04401579
5. Domling and Gao. Chemistry and Biology of SARS-CoV-2, (2020) Chem., https://doi.org/10.1016/j.chempr.2020.04.02
6. Zhang, et al. Angiotensin- converting enzyme 2 (ACE2) as a SARS- CoV-2 receptor: molecular mechanisms and potential therapeutic target. (2020). Intensive Care Med., 46, 586–5905.
7. Hoffmann, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. (2020) Cell, 181, 271–280.
8. Pipeline Review. APEIRON’s respiratory drug product to start pilot clinical trial to treat coronavirus disease COVID-19 in China. Pipeline Review https://pipelinereview.com/index.php/2020022673884/Proteins- and- Peptides/APEIRONs- respiratory- drugproduct-to- start- pilot- clinical- trial- to- treat- coronavirusdisease- COVID-19- in- China.html (2020).
9. Marcus, et al. Narrowing the gap in life expectancy for HIV+ compared with HIV- individuals. Conference on Retroviruses and Opportunistic Infections (CROI), Boston, abstract 54, 2016.
Other Suggested Reading:
Li, et al. Therapeutic options for the 2019 novel coronavirus (2019-nCoV). (2020) Nat. Rev. Drug Discov. 19, 149–150.
Tay, et al. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. (2020) 20(6):363-374. doi: 10.1038/s41577-020-0311-8. Epub 2020 Apr 28.
Oberfield, et al. SnapShot: COVID-19. (2020) Cell 181, May 14, 2020 doi.org/10.1016/j.cell.2020.04.013.
email info@multispaninc.com
Let’s discuss how MultiScreen™ cell membranes can uniquely empower your program.

Recent Comments