Covid-19 Resources and Technology
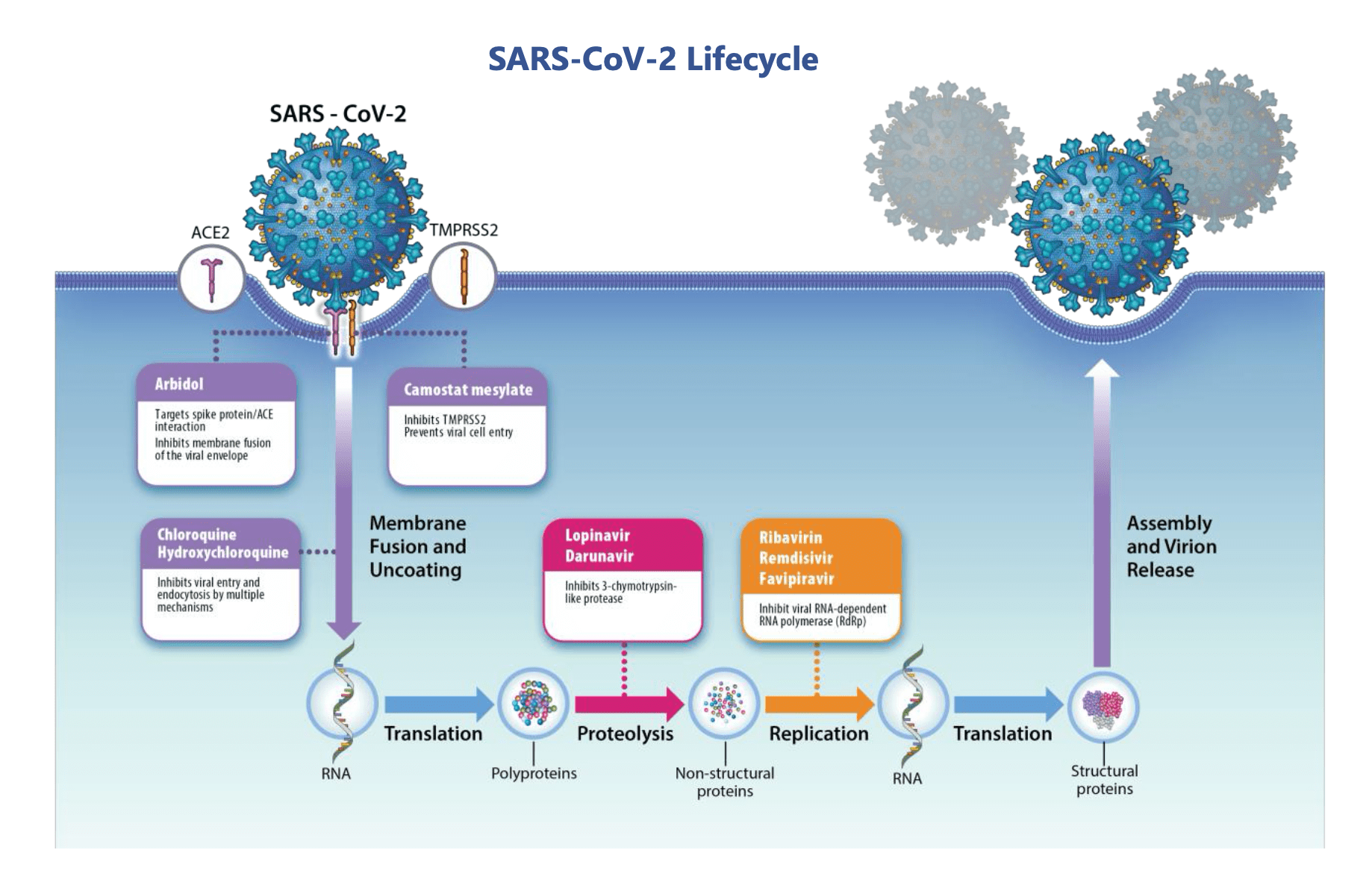
“Three coronaviruses have emerged over the last 20 years as serious human pathogens: SARS-CoV was identified as the causative agent in an outbreak in 2002-2003 outbreak, Middle East respiratory syndrome (MERS) CoV emerged in 2012 and the novel coronavirus SARS-CoV-2 emerged in 2019-2020. These are all enveloped, single-stranded positive-sense RNA viruses whose genomes and protein structures are highly conserved” (Alexander SP, Ball JK, Tsoleridis T).
Alexander SP, Ball JK, Tsoleridis T. Coronavirus (CoV) proteins (version 2020.5) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide to Pharmacology CITE. 2020; 2020(5). Available from: https://doi.org/10.2218/gtopdb/F80/2020.5.
Cell Lines and Membranes
| Receptor Family | Receptor | Species | Parental | Stable Cell Lines | Division-Arrested Cells | Membranes |
|---|---|---|---|---|---|---|
| SARS-CoV-2 | ACE2 | human | HEK293T | C2006 | DC2006 | MC2006 |
| TMPRSS2-ACE2 | human | HEK293T | C2007 | DC2007 | MC2007 |
Meeting The Need For Accelerated Covid Antiviral And Vaccine Development
The rapid emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or COVID-19 has substantially impacted both human health and the global economy.
Helping Make a Difference
Control and eradication of SARS-Cov-2 will require advanced antivirals and/or a protective vaccine – development of both of which depends on improved knowledge of the virus pathophysiology, identification and therapeutic exploitation of the vulnerabilities of the virus, and characterization of the components of protective immunity.
A variety of assays are needed ranging from general infectivity to highly specific viral pathways including binding, entry, enzymatic activity, maturation, and packaging.
Multispan has a proven record of complex cell engineering and assay development. Ensure the advancement of your SARS-CoV-2 drug and vaccine development efforts by harnessing the knowledge and expertise of their scientific teams. Contact Multispan today to discuss the design and implementation of your COVID assay.