Multiscreen GTPγS Functional Assays
Identifying novel agonists and antagonists to modulate GPCR activity for developing new therapeutics remains integral to drug discovery efforts. Unlike traditional radioligand binding assays, GTPγS assays can distinguish between antagonist and agonist activity. It can also dissect nuanced MOAs to characterize drug candidate such as inverse agonism, partial antagonism/agonism, and positive and negative allosteric modulation (PAM and NAM). GTPγS assay is an integral part of the MULTISCREENTM GPCR assay platform, utilizing high-quality HTS-ready membrane and cell lines to provide answers to questions.
Screening for novel GPCR ligands for drug discovery
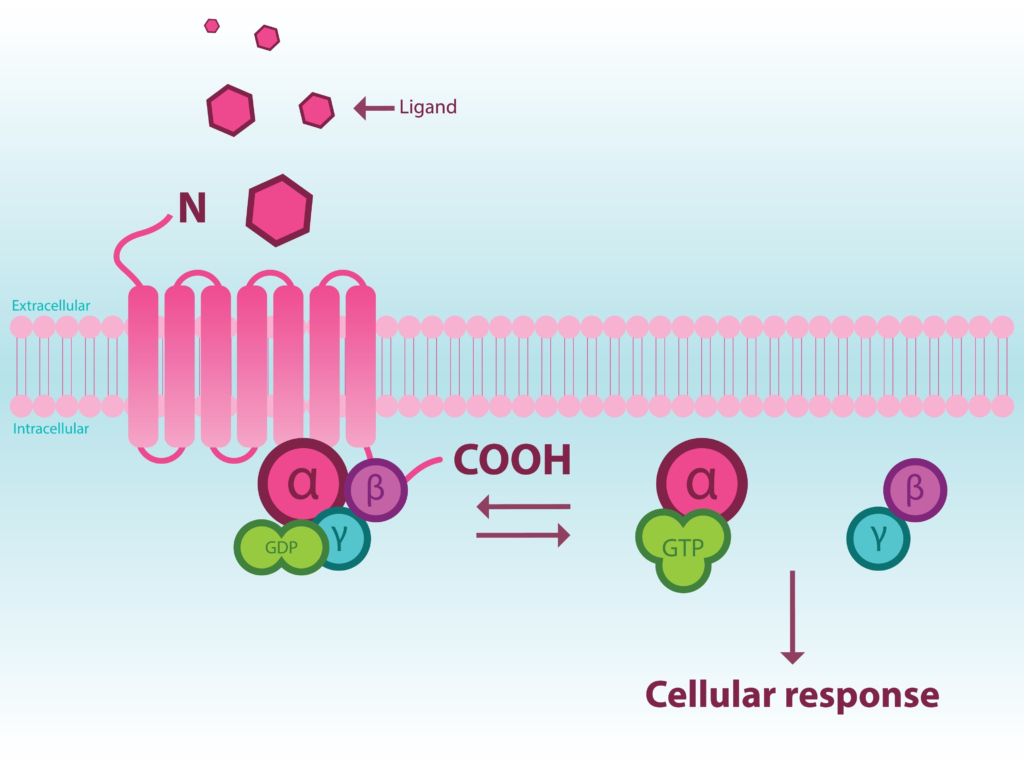
G protein-coupled receptors (GPCRs) are a diverse group of seven-transmembrane receptors whose activation via an equally diverse set of extracellular ligands that promote cell signaling through a complex network of intracellular pathways. GPCRs are essential for a host of physiological and pathological processes and their involvement in a multitude of diseases (e.g., neurological disorders [1-2], cancer [3-4], inflammatory diseases [5-6]) has led them to be the targets of more than 30% of all FDA-approved drugs [7].
Given their importance as therapeutic targets, screening for novel GPCR ligands continues to be a focus of many drug discovery efforts. Historically, screening has been accomplished via radioligand binding assays using receptor-containing membrane preparations where ligand binding affinity can be measured. Radioligand binding assays have a few limitations. Most importantly, they cannot identify the functional response to ligand binding, i.e., radioligand binding cannot distinguish between an agonistic and antagonistic ligand. Second, they are done using membrane preparations that cannot reflect physiological responses in intact cells. Functional assays using intact cells offer the ability to ascertain whether a compound serves as an antagonist or agonist as well as measure responses under physiological conditions. Several GPCR functional assays have been developed [8].
G protein-dependent assays to measure receptor activity besides ligand binding can utilize the quantification of GTPγS binding, cAMP production, intracellular Ca2+ concentration, IP1/3 production, or GRK2 phosphorylation, among other functional outputs (Figure 1). Because of their dependence on G proteins, these second messenger assays are suitable for subsets of GPCRs based on the G proteins they associate with, allowing for tailored specificity. For instance, cAMP production can be utilized for GPCRs that associate with Gαs and Gαi/o G proteins, while Ca2+ would be suitable for Gαq-associated GPCRs. G protein-independent measurements can include such responses as β-Arrestin recruitment and are suitable for all GPCRs.
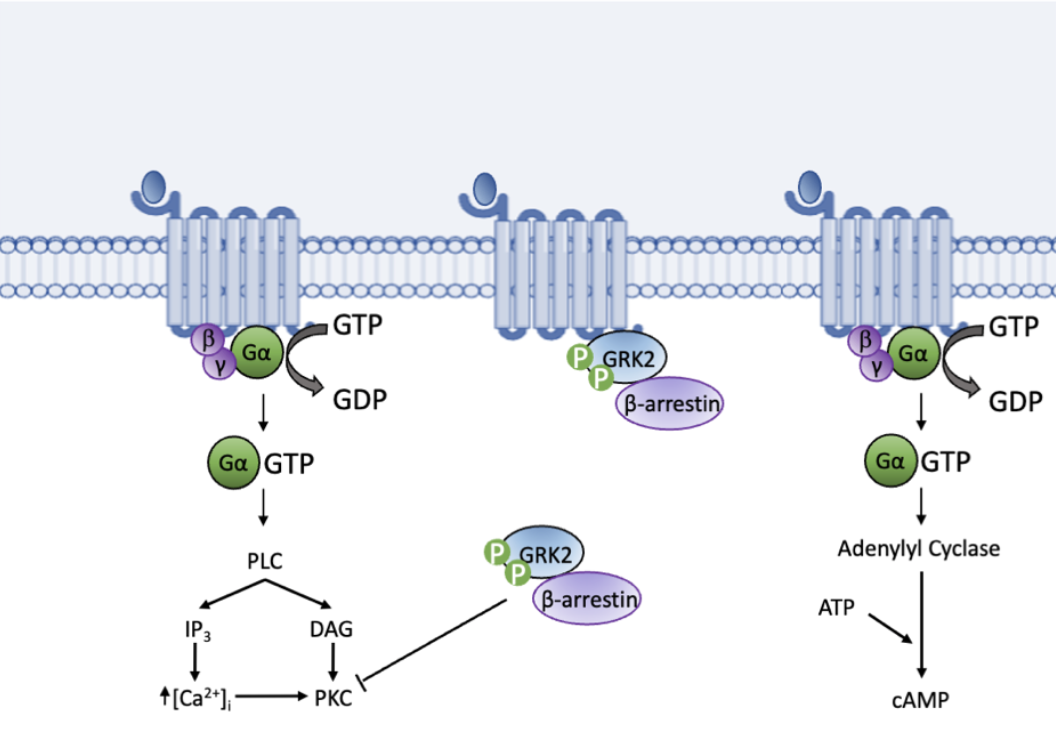
Figure 1. Functional assays make use of downstream signaling processes, including those shown above, following GPCR ligand binding to determine ligand specificity and function
GTPγS binding assays
GTPγS binding assays have served to measure kinetics, including maximal (Emax) and half maximal (EC50) effective concentrations of known agonists and screen for novel agonists and antagonists. Classically these assays have utilized a radiolabeled GTPγS, a non-hydrolyzable analog of GTP, most commonly [35S] GTPγS, which is incubated with receptor-containing membrane preparations. [35S] GTPγS replaces endogenous GTP, binding to the Gα subunit upon receptor activation (Figure 2). [35S] GTPγS can then be measured as a readout for ligand-mediated GPCR activity [9].
GTPγS functional assays allow for screening and characterization of nuanced mechanisms of action (MOAs) in drug discovery, distinguishing between agonism and antagonism, partial agonism/antagonism, positive (PAM) or negative (NAM) allosteric modulators, or inverse agonism. Inverse agonism occurs when a ligand binds the agonist binding site, inhibiting agonism via blocking agonist binding and antagonizing constitutive signaling. As the name implies, partial agonists occupy the agonist binding site but only result in partial agonism. Similarly, partial antagonism does not fully inhibit receptor signaling. Allosteric modulators can bind the receptor, modifying receptor responses to agonists. These can be either positive (PAM) or negative (NAM) allosteric modulators. GTPγS functional assays can precisely and reproducibly dissect these specific MOAs that are not accessible by the radioligand binding and some functional assays.
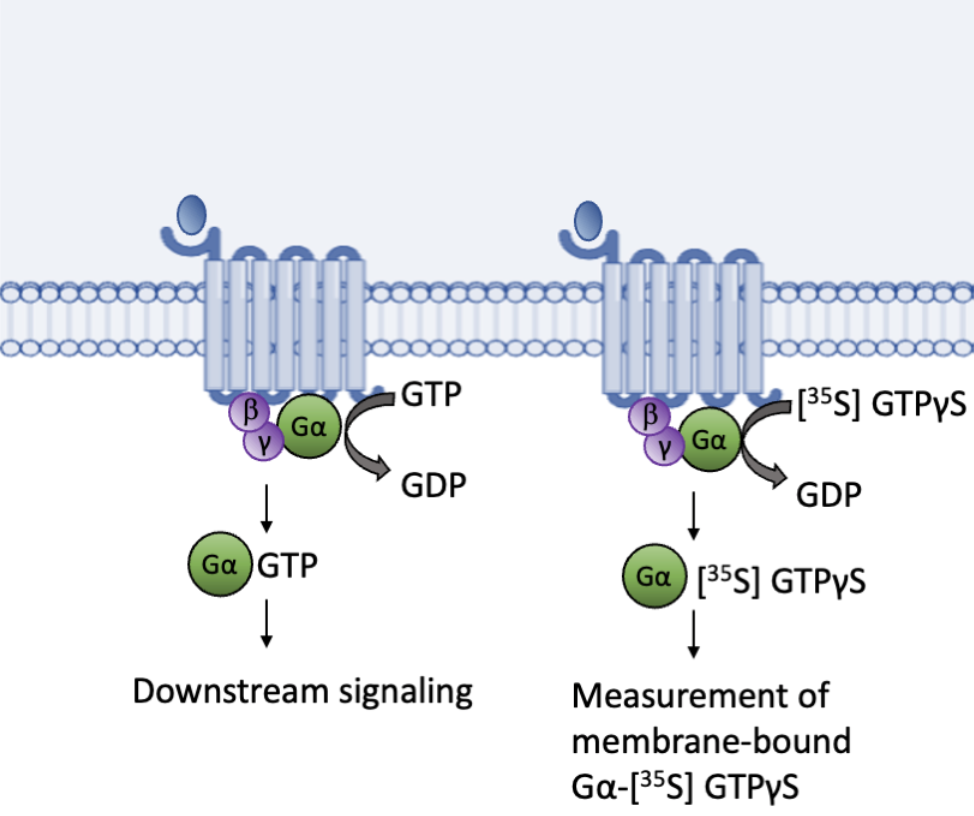
Figure 2. GTPγS binding assays utilize the endogenous function of guanine nucleotide exchange factors (GEFs) to replace GDP with a radiolabeled non-hydrolyzable [35S] GTPγS
Compared to functional assays, GTPγS assay also presents some advantages. In addition to GTPγS binding assays, downstream signaling-based readouts including the production of cAMP or IP1/3, changes in Ca2+ concentration, phosphorylation of G protein-coupled receptor kinase 2 (GRK2), and β-arrestin recruitment can be used as functional measurements of receptor activation (Figure 1). These assays allow for receptor specificity by selecting the most suitable second messenger as the functional readout. However, there are advantages to using the GTPγS functional assay. First, guanine nucleotide exchange is more proximal to the receptor than second messengers and thus is not amplified by downstream reactions. Therefore, GTPγS binding assays are more reproducible and consistent in measuring nuanced MOAs including NAM, PAM, and partial antagonism, amenable for repeated structure-activity relationship (SAR) lead optimization assays.
MULTISCREENTM GTPγS HTS Services
The GTPγS assay has been a staple in Multispan’s GPCR assay platform. Screening thousands or hundreds of thousands of compounds using GTPγS assay can be time-consuming and slow. High-throughput screening (HTS) to accelerate drug discovery is made accessible by advancements in robotics, data processing software, and detectors. Reproducible and reliable data produced quickly and accurately saves time and money, and helps avoid wasted research funding and efforts.
Using an extensive library of over 500 validated GPCR stable cell lines, Multispan can provide a reliable source of high-quality membrane preparations for GTPγS HTS. When needed, Multispan can also generate custom membrane preparations to meet any screening needs. We regularly conduct weekly SAR lead optimization campaigns utilizing GTPγS functional assays, providing the most reliable and reproducible screening results. Combined with the unprecedented customization available from Multispan, rapid drug discovery screening and characterization are within reach, to help drive the IND-enabling candidate delivery with confidence.
References
- Thathiah A, De Strooper B. The role of G protein-coupled receptors in the pathology of Alzheimer’s disease. Nat Rev Neurosci. 2011 Feb;12(2):73-87. doi: 10.1038/nrn2977. PMID: 21248787.
- Huang Y, Todd N, Thathiah A. The role of GPCRs in neurodegenerative diseases: avenues for therapeutic intervention. Curr Opin Pharmacol. 2017 Feb;32:96-110. doi: 10.1016/j.coph.2017.02.001. Epub 2017 Mar 10. PMID: 28288370.
- Insel PA, Sriram K, Wiley SZ, Wilderman A, Katakia T, McCann T, Yokouchi H, Zhang L, Corriden R, Liu D, Feigin ME, French RP, Lowy AM, Murray F. GPCRomics: GPCR Expression in Cancer Cells and Tumors Identifies New, Potential Biomarkers and Therapeutic Targets. Front Pharmacol. 2018 May 22;9:431. doi: 10.3389/fphar.2018.00431. PMID: 29872392; PMCID: PMC5972277.
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007 Feb;7(2):79-94. doi: 10.1038/nrc2069. PMID: 17251915.
- Feng Z, Sun R, Cong Y, Liu Z. Critical roles of G protein-coupled receptors in regulating intestinal homeostasis and inflammatory bowel disease. Mucosal Immunol. 2022 May;15(5):819-828. doi: 10.1038/s41385-022-00538-3. Epub 2022 Jun 22. PMID: 35732818.
- Sun L, Ye RD. Role of G protein-coupled receptors in inflammation. Acta Pharmacol Sin. 2012 Mar;33(3):342-50. doi: 10.1038/aps.2011.200. Epub 2012 Feb 27. PMID: 22367283; PMCID: PMC4085652.
- Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE. Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov. 2017 Dec;16(12):829-842. doi: 10.1038/nrd.2017.178. Epub 2017 Oct 27. PMID: 29075003; PMCID: PMC6882681.
- Zhang R, Xie X. Tools for GPCR drug discovery. Acta Pharmacol Sin. 2012 Mar;33(3):372-84. doi: 10.1038/aps.2011.173. Epub 2012 Jan 23. PMID: 22266728; PMCID: PMC3312097.
- Vasavda C, Zaccor NW, Scherer PC, Sumner CJ, Snyder SH. Measuring G-protein-coupled Receptor Signaling via Radio-labeled GTP Binding. J Vis Exp. 2017 Jun 9;(124):55561. doi: 10.3791/55561. PMID: 28654029; PMCID: PMC5608332.

Recent Comments